Amide
|
Read other articles:

CrawlingSingel oleh Linkin Parkdari album Hybrid TheorySisi-BPapercut (Langsung dari BBC)Dirilis14 November 2000[1]DirekamNew Orleans, Louisiana, 1998-1999Genre Nu metal[2][3] rap metal[4] rap rock[3] Durasi3:29LabelWarner Bros.PenciptaLinkin ParkProduserDon GilmoreKronologi singel Linkin Park One Step Closer (2000) Crawling (2000) Papercut (2001) Video musikCrawling di YouTube Crawling adalah sebuah lagu oleh grup musik rok Amerika Linkin Park. Ini ada...

Partai Demokrat Konstitusional JepangConstitutional Democratic Party of Japan立憲民主党 atau 立民党 Rikken MinshutōKetua umumKenta IzumiSekretaris JenderalChinami NishimuraKetua DeputiSeiji OsakaKetua Biro KebijakanAkira NagatsumaDibentuk2 Oktober 2017; 6 tahun lalu (2017-10-02)Digabungkan dariPartai Demokrat untuk Rakyat (mayoritas)Partai Demokrat Sosial (mayoritas)Dipisah dariPartai Demokrat (2016)Kantor pusat2-12-4 Fuji Building 3F, Hirakawa-cho, Chiyoda-ku, Tokyo, ...

У Вікіпедії є статті про інші географічні об’єкти з назвою Пенфілд. Місто Пенфілдангл. Penfield Координати 43°09′40″ пн. ш. 77°26′48″ зх. д. / 43.16111111113877286° пн. ш. 77.44666666669478161° зх. д. / 43.16111111113877286; -77.44666666669478161Координати: 43°09′40″ пн. ш. 77°26′48″ з�...

Indonesian rice and vegetable dish Nasi pecelNasi pecelCourseMain coursePlace of originIndonesiaRegion or stateEast Java, Central Java, YogyakartaServing temperatureRoom temperatureMain ingredientsRice with vegetables in peanut sauce Nasi pecel also known as Sega pecel in Javanese is an Indonesian rice dish from Java served with pecel (cooked vegetables and peanut sauce).[1] The vegetables are usually kangkung or water spinach, long beans, cassava leaves, papaya leaves, and in East Ja...
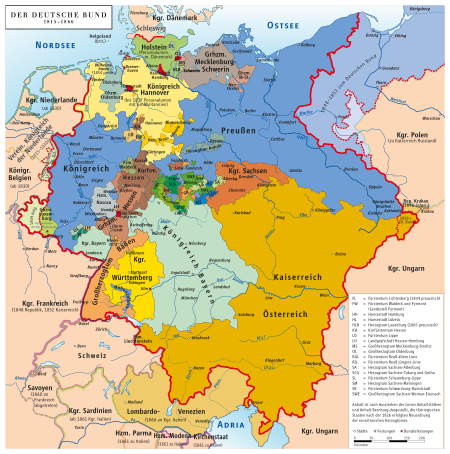
„An mein Volk und an die Deutsche Nation“ wohl hauptsächlich von Heinrich Alexander von Arnim-Suckow verfasst[1], 21. März 1848, Einblattdruck auf Papier Exemplar des Deutschen Historischen Museums (Berlin): 42 cm × 62,4 cm.[2] und Exemplar des Stadtarchivs Paderborn: 35 cm × 22 cm[3] Vorlage:Infobox Gemälde/Wartung/Museum „An mein Volk und an die deutsche Nation“ ist eine Proklamation vom 21. März 1848, die im Zusammenhang mit der Berliner Märzrevo...

Míchel Informações pessoais Nome completo Miguel Ángel Sánchez Muñoz Data de nascimento 30 de outubro de 1975 (48 anos) Local de nascimento Madrid, Espanha Informações profissionais Posição Meia Clubes de juventude Rayo Vallecano Clubes profissionais1 Anos Clubes Jogos e gol(o)s Rayo Vallecano 1 Partidas e gols pelos clubes profissionaiscontam apenas partidas das ligas nacionais.Atualizadas até 3 de março de 2018. Miguel Ángel Sánchez Muñoz (Madrid, 30 de outubro de 1...

Pontifical Filipino CollegePontificio Collegio FilippinoDalubhasaang Pilipinong PontipikalLatin: Seminarii Sanctae Mariae de Pace, et in Collegio S. Bon VoyageMottoSacerdotes Domini VocabiminiMotto in English...(ye) shall be named the Priests of the LORD... (Isaiah 61:6)TypeRoman Catholic Seminary; Continuing Formation Institute; ResidenceEstablishedJune 29, 1961 (62 years ago)RectorRev. Fr. Gregory Ramon D. Gaston, S.Th.D.Location490 Via Aurelia, Rome, Italy (on property subject i...

Thurgood Marshall Supreme Court nominationMarshall (left) and President Johnson meet in the Oval Office of the White House on June 13, 1967 before the announcement of the nominationNomineeThurgood MarshallNominated byLyndon B. Johnson (president of the United States)SucceedingTom C. Clark (associate justice)Date nominatedJune 13, 1967Date confirmedAugust 30, 1967OutcomeApproved by the U.S. SenateSenate Judiciary Committee voteVotes in favor11Votes against5ResultReported favorablySenate confir...

Filipino fast food restaurant chain This article is about the fast food chain. For the company which owns the brand and other fast food chains, see Jollibee Foods Corporation. Not to be confused with Jubilee. This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) The article's lead section may need to be rewritten. Please help improve the lead and read the lead layout guide. (September 2023) (Le...

Pusat PeneranganTentara Nasional IndonesiaLambang Tentara Nasional IndonesiaNegara IndonesiaCabang Tentara Nasional IndonesiaTipe unitBadan Pelaksana Pusat TNIBagian dariTentara Nasional IndonesiaSitus webwww.tni.mil.idTokohKepalaLaksamana Muda TNI Julius WidjojonoWakil KepalaBrigadir Jenderal TNI Teguh Pudji Rahardjo Pusat Penerangan Tentara Nasional Indonesia, disingkat (Puspen TNI) adalah Badan Pelaksana Pusat ditingkat markas besar Tentara Nasional Indonesia yang berkedudukan langsun...

Some of this article's listed sources may not be reliable. Please help this article by looking for better, more reliable sources. Unreliable citations may be challenged or deleted. (November 2022) (Learn how and when to remove this template message) Frankie CenaBorn (1991-09-11) September 11, 1991 (age 32)Burnaby, British Columbia, CanadaAlma materUniversity of British ColumbiaOccupationFormer pageant winnerHeight1.68 m (5 ft 6 in)Beauty pageant titleholderTitleMr. Wo...

American musician and actor Not to be confused with Robert Shwartzman. Robert SchwartzmanSchwartzman performing in 2008BornRobert Coppola Schwartzman (1982-12-24) December 24, 1982 (age 40)Los Angeles, California, U.S.Other namesRobert CarmineOccupation(s)Actor, musician, directorYears active1998–presentSpouse Zoey Grossman (m. 2017)ParentsJack Schwartzman (father)Talia Shire (mother)Relatives Jason Schwartzman (brother) John Schwartzman (half-b...

Kantor Pos dan Telegraf Medan dan air mancur Nienhuys. Kantor Pos Medan, dan air mancur Nienhuys yang sedang direnovasi Kantor Pos Medan adalah kantor pos besar di Medan, Indonesia. Dibuka pada tahun 1911, kantor pos ini adalah salah satu bangunan bersejarah yang hingga kini masih berdiri di Medan. Bangunan ini masih tetap mempertahankan fungsinya hingga kini. Letaknya di pusat kota Medan, tepatnya di seberang Lapangan Merdeka dan Hotel Dharma Deli. Di depannya terdapat air mancur yang didedi...

American roller derby skater (1935–1997) This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Joan Weston – news · newspapers · books · scholar · JSTOR (October 2009) (Learn how and when to remove this template message) JOAN WESTON, Madison Square Garden, November 1986 Joan Weston or Joanie Weston (January 20, ...

Public schoolJuanita High SchoolLocation10601 Northeast 132nd StreetKirkland, WashingtonCoordinates47°42′57″N 122°11′58″W / 47.7157259°N 122.1995564°W / 47.7157259; -122.1995564InformationTypePublicEstablishedSeptember 4, 1971PrincipalKelly ClappStaff140Enrollment1,709 (2022-2023)Student to teacher ratio12.14Color(s)Red, White, and Navy BlueMascotRavenRivalLake Washington High School and Inglemoor High SchoolWebsitejhs.lwsd.orgJuanita High School is a high ...

Wilayah yang didominasi oleh Mozaffarid pada puncak kejayaannya Makam Djalâl ad-Dîne Chah Chodja (1364-1384) di Syiraz Dinasti Muzaffarid (Persia: مظفریان) merupakan sebuah dinasti Persia[1][2] yang berkuasa di Iran setelah pecahnya Ilkhanat pada abad ke-14. Pada puncak kejayaan, mereka memerintah sebuah kerajaan yang terdiri dari Azerbaijan Iran, Persia Tengah, dan Irak Persia. Daftar penguasa Muzaffarid Mubariz al-Din Muhammad (1314–1358) Shah Shoja (1358-1364...

Suite of software including Final Cut Pro This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Final Cut Studio – news · newspapers · books · scholar · JSTOR (January 2016) (Learn how and when to remove this template message) Final Cut StudioDeveloper(s)Apple Inc.Operating systemMac OS XTypeNon-linear editing sys...

Major river in Asia This article is about the river. For other uses, see Ganges (disambiguation), Ganga (disambiguation), and Ganga (goddess). GangesThe Ganges in VaranasiMap of the combined drainage basins of the Ganges (yellow), Brahmaputra (violet) and Meghna (green)EtymologyGanga (goddess)LocationCountryIndia (as Ganga), Bangladesh (as Padma)CitiesUttarakhand: Rishikesh, Haridwar Uttar Pradesh: Bijnor,Fatehgarh, Kannauj, Hardoi, Bithoor, Kasganj, Kanpur, Prayagraj, Mirzapur, Varanasi, Gha...

Lublin District in Lublin Voivodeship, PolandRuryLublin DistrictView upon allotments and apartments by Nadbystrzycka StreetLocation of Rury within LublinCoordinates: 51°14′19″N 22°30′59″E / 51.23861°N 22.51639°E / 51.23861; 22.51639Country PolandVoivodeshipLublinCounty/CityLublinArea • Total3.61 km2 (1.39 sq mi)Population (2016) • Total30,093[1]Time zoneUTC+1 (CET) • Summer (DST)UTC+2 (CES...

This article is about the Nintendo 3DS game. For the main EA game series, see Madden NFL. The factual accuracy of parts of this article (those related to article) may be compromised due to out-of-date information. Please help update this article to reflect recent events or newly available information. (March 2012) 2011 video gameMadden NFL FootballLogo for Madden NFL FootballDeveloper(s)EA SportsPublisher(s)EA SportsSeriesMadden NFLPlatform(s)Nintendo 3DSReleaseNA: March 22, 2011EU: March 25,...








