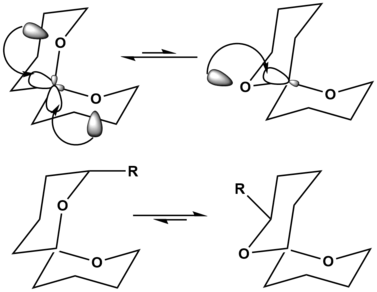
Anomeric effect
|
Read other articles:

Municipality in Valencian Community, SpainSimat de la ValldignaMunicipality Coat of armsSimat de la ValldignaLocation of Simat de la Valldigna in the Province of ValenciaShow map of Province of ValenciaSimat de la ValldignaLocation of Simat de la Valldigna in the Valencian CommunityShow map of Valencian CommunitySimat de la ValldignaLocation of Simat de la Valldigna in SpainShow map of SpainCoordinates: 39°2′40″N 0°18′46″W / 39.04444°N 0.31278°W / 39.04444;...
TrueVisionsJenisPublik: (SET:TRUE)IndustriTelevisi berbayarDidirikan1990Kantorpusat118/1 Tipco Building, Rama XI Road, Sam Sen Nai, Payathai, Bangkok 10400, ThailandTokohkunciSoopakij Chearavanont (Chairman) Supachai Chearavanont (CEO)ProdukSatelit digital dan TV KabelSitus webwww.truevisionstv.com TrueVisions adalah operator televisi satelit kabel terkemuka di Thailand. Kini dimiliki oleh True Corporation, perusahaan ini sebelumnya dikenal sebagai United Broadcasting Corporation (UBC),...

Voor het gelijknamige metrostation, zie Hyde Park Corner (metrostation). Hyde Park Corner, Thomas Shotter Boys, 1842 Hyde Park Corner ligt in de zuidoostelijke hoek van Hyde Park in Londen. Het is een belangrijk verkeersknooppunt waar Park Lane, Knightsbridge, Piccadilly, Grosvenor Place en Constitution Hill samenkomen. Een stelsel van voetgangerstunnels onder het straatniveau biedt wandelaars toegang tot het park. Na de Tweede Wereldoorlog werd Park Lane verbreed tot een grote verkeersader m...

Cet article est une ébauche concernant l’architecture ou l’urbanisme. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Porte cochère équipée de chasse-roues métalliques. Une porte cochère (appelé aussi passage cocher ou portail cocher) est une porte dans la façade d'un bâtiment (portail d'hôtel particulier, de manoir, de bâtiment public), par laquelle les véhicules peuvent passer (une entrée de ga...

Eurovision Song Contest 2014Country MaltaNational selectionSelection processMalta Eurovision Song Contest 2014Selection date(s)Semi-final:7 February 2014Final:8 February 2014Selected entrantFirelightSelected songComing HomeSelected songwriter(s)Richard MicallefFinals performanceSemi-final resultQualified (9th, 64 points)Final result23rd, 32 pointsMalta in the Eurovision Song Contest ◄2013 • 2014 • 2015► Malta participated in the Eurovision Song ...

Dieser Artikel behandelt die Gemeinde in Thüringen. Siehe auch: Arentshaus bzw. Arnshausen. Wappen Deutschlandkarte 51.3766666666679.9716666666667202Koordinaten: 51° 23′ N, 9° 58′ O Basisdaten Bundesland: Thüringen Landkreis: Eichsfeld Verwaltungsgemeinschaft: Hanstein-Rusteberg Höhe: 202 m ü. NHN Fläche: 5,79 km2 Einwohner: 1016 (31. Dez. 2022)[1] Bevölkerungsdichte: 175 Einwohner je km2 Postleitzahl: 37318 Vorwa...

Season of American Football League team the Boston Patriots 1965 Boston Patriots seasonOwnerBilly SullivanHead coachMike HolovakHome fieldFenway ParkResultsRecord4–8–2Division place3rd AFL EasternPlayoff finishDid not qualifyAFL All-StarsDT Houston AntwineLB Nick BuonicontiDE Bob DeeC Jon MorrisUniform ← 1964 Patriots seasons 1966 → The 1965 Boston Patriots season was the franchise's 6th season in the American Football League. The Patriots ended the season with ...

British cellist (born 1958) Steven IsserlisCBEIsserlis in 2018Background informationBorn (1958-12-19) 19 December 1958 (age 64)London, EnglandGenresClassicalOccupation(s)MusicianauthorInstrument(s)CelloYears active1977–present[1]LabelsHyperionRCA Red SealBISVirgin ClassicsWebsitestevenisserlis.comMusical artist Steven Isserlis CBE (born 19 December 1958) is a British cellist. An acclaimed soloist, chamber musician, educator, writer and broadcaster, he is widely regarded as one ...

Destroyed memorial in New York City 1993 World Trade Center Bombing Memorial FountainThe names of the victims of the 1993 bombing, on the Reflecting Absence memorial (original 1993 memorial not pictured)ArtistElyn ZimmermanCompletion date1995Conditiondestroyed in the September 11, 2001 attacks The 1993 World Trade Center Bombing Memorial was created to commemorate the six lives lost during the February 26, 1993, bombing of the World Trade Center in New York City. The memorial was commissioned...

Equestrian statue by Adamo Tadolini Simon BolivarThe original statue in Plaza Bolívar in Lima (2018)ArtistAdamo TadoliniYear1859 (1859) (Lima)TypeSculptureMediumBronzeSubjectSimón BolívarLocationLima, Peru (1850s); Caracas, Venezuela (1870s); San Francisco, United States of AmericaCoordinates12°02′53″S 77°01′35″W / 12.04808°S 77.02650°W / -12.04808; -77.02650 Simon Bolivar, also known as General Bolivar,[1] is a bronze equestrian statue of Sim

Nicholas ChoiInformasi pribadiNama lahirNicholas Edward ChoiLahir20 Januari 1993 (umur 30)Hong KongSenjataFloretTangantangan kananTinggi badan175 m (574 ft 2 in)Berat badan65 kg (143 pon)Peringkat FIEperingkat saat ini Rekam medali Floret putra Mewakili Hong Kong Kejuaraan Asia 2012 Wakayama Tim 2013 Shanghai Tim Nicholas Edward Choi (Hanzi: 崔浩然; Jyutping: ceoi1 hou5 jin4; lahir 20 Januari 1993) adalah seorang atlet anggar floret asal Hong Kon...

This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article may contain excessive or irrelevant examples. Please help improve the article by adding descriptive text and removing less pertinent examples. (May 2013) This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and rem...

American politician (born 1964) Sarah PalinPalin in 20219th Governor of AlaskaIn officeDecember 4, 2006 – July 26, 2009LieutenantSean ParnellPreceded byFrank MurkowskiSucceeded bySean ParnellChair of the Alaska Oil and Gas Conservation CommissionIn officeFebruary 19, 2003 – January 23, 2004GovernorFrank MurkowskiDeputyMike BillRandy RuedrichDaniel SeamountPreceded byCamille TaylorSucceeded byJohn NormanMayor of WasillaIn officeOctober 14, 1996 – October 14, 20...

American restaurant chain This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Penn Station restaurant – news · newspapers · books · scholar · JSTOR (August 2008) (Learn how and when to remove this template message) Penn Station Inc.Penn Station location in Springboro, OhioTrade namePenn Station East Coast S...

الدوري الألماني الدرجة الثانية 1986–87 تفاصيل الموسم الدوري الألماني الدرجة الثانية النسخة 13 البلد ألمانيا التاريخ بداية:26 يوليو 1986 نهاية:14 يونيو 1987 المنظم الاتحاد الألماني لكرة القدم مباريات ملعوبة 380 عدد المشاركين 20 أهداف مسجلة 1201 الحضور ال�...

Association football club in Bucharest This article is about the Steaua București football team which competes in the Liga II. For the team previously named FC Steaua București which plays in the Liga I, see FCSB. For the parent multi-sport club, see CSA Steaua București. For other uses, see Steaua București (disambiguation). The neutrality of this article is disputed. Relevant discussion may be found on the talk page. Please do not remove this message until conditions to do so are met. (...

Species of rodent Bank vole Conservation status Least Concern (IUCN 3.1)[1] Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Chordata Class: Mammalia Order: Rodentia Family: Cricetidae Subfamily: Arvicolinae Genus: Clethrionomys Species: C. glareolus Binomial name Clethrionomys glareolus(Schreber, 1780) Range of bank vole Synonyms Myodes glareolus Evotomys glareolus The bank vole (Clethrionomys glareolus) is a small vole with red-brown fur and some grey ...

Zeki MajedZeki Majed in Vienna.BornZeki Anwar Majed (1996-11-13) 13 November 1996 (age 27)Sofia, BulgariaNationalityKurdishOccupation(s)Filmmaker, poet Zeki Majed (born 13 November 1996) is a Kurdish filmmaker and poet. He was born and raised in Sofia, Bulgaria. Biography His first poem, Eyes Never Dry, won first prize at Pendle War Poetry Competition. He wrote multiple love poems, praising women. A poem he wrote, Brave Woman,[1] was read at St Margaret's, Westminster by Glenys K...

Mountain marathon in North Wales Snowdonia MarathonLogo of the Snowdonia MarathonDateOctoberLocationSnowdonia National Park, WalesEvent typeMountainDistanceMarathonEstablished1982Official sitesnowdoniamarathon.co.uk The Snowdonia Marathon, known as Marathon Eryri from 2023, is a marathon in Snowdonia (Eryri), North Wales. It was established in 1982 as an alternative to city and town races. The route makes a circumnavigation of the Snowdon massif, starting and finishing at Llanberis.[1]...

Lolah-ye Gaw Kush(Dari: لولۀ گاو کش)Lolah-ye Gaw KushLocation in the Hindu Kush Highest pointElevation2,226 m (7,303 ft)Parent peakHindu KushCoordinates35°42′34.5″N 69°23′29.3″E / 35.709583°N 69.391472°E / 35.709583; 69.391472GeographyLocationTagab, Kapisa, AfghanistanParent rangeHindu Kush Lolah-ye Gaw Kush (Dari: لولۀ گاو کش is a mountain in Afghanistan. vteKapisa ProvinceCapital: Mahmud-i-RaqiDistricts Alasay Hesa Awal Ko...