Meerwein–Ponndorf–Verley reduction
| |||||||||||||||||||||||||
Read other articles:

French mathematician and physicist Yvonne Choquet-BruhatYvonne Choquet-Bruhat in 2006Born (1923-12-29) 29 December 1923 (age 99)Lille, FranceNationalityFrenchAlma materÉcole Normale SupérieureFrench National Centre for Scientific ResearchKnown forWell-posedness of the vacuum Einstein EquationsAwardsGrand Officier of the Légion d'honneur Elected to the French Academy of Sciences Elected to the American Academy of Arts and SciencesScientific careerFieldsMathematics, physicsIns...

Santo KenelmMeninggal17 Juli 811Clent HillsDihormati diGereja Katolik RomaTempat zairahWinchcombPesta17 Juli Gereja St. Kenelm, Clent Hills, Worcestershire. Mata air St. Kenelm di sebelah gereja di Clent Hills, (salah satu hulu Sungai Stour.) Kenelm atau Cynehelm merupakan seorang Santo Anglo-Saxon. Biografi Kehidupan Kenelm adalah bahan hagiografi yang ditulis pada sekitar pertengahan abad kesebelas, Vita et Miracula Sancti Kenelmi, mungkin ditulis oleh Goscelin atas permintaan para biarawan...

Aquiles en Esciros Achille in Sciro Pasquale AnfossiGénero Ópera seriaActos Tres actosBasado en Achille in SciroPublicaciónIdioma ItalianoMúsicaCompositor Pasquale AnfossiPuesta en escenaLugar de estreno Teatro de la Torre Argentina (Roma)Fecha de estreno 1774Personajes véase PersonajesLibretista Pietro Metastasio[editar datos en Wikidata] Aquiles en Esciros (Achille in Sciro) es el título de una ópera seria que el italiano Pietro Metastasio (1698 – 1782) escribió com...

لمعانٍ أخرى، طالع إرنست هوفمان (توضيح). هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (نوفمبر 2018) إرنست هوفمان (بالألمانية: Ernst Hofmann) معلومات شخصية اسم الولادة (بالألمانية: Ernst Karl Heinrich Hofmann) الميلاد...

2006 novel by Helen Dunmore This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: The Tide Knot – news · newspapers · books · scholar · JSTOR (August 2015) (Learn how and when to remove this template message) The Tide Knot AuthorHelen DunmoreCountryUnited KingdomLanguageEnglishGenreChildren's NovelPublisherHarperC...

Едвін Гарріс Колберт Народився 28 вересня 1905(1905-09-28)[1][2][3]Айова, СШАПомер 15 листопада 2001(2001-11-15)[2][4] (96 років)Флегстафф, Аризона, США[5]Країна СШАДіяльність палеонтологГалузь палеонтологіяAlma mater Колумбійський університет, Університет Небраски-�...

1940 British filmCharley's (Big-Hearted) AuntDirected byWalter FordeWritten byBrandon Thomas (original play)J.O.C. Orton (screenplay)Marriott Edgar (screenplay)Ralph Smart (screenplay)Produced byEdward BlackStarringSee belowCinematographyArthur CrabtreeEdited byR.E. DearingMusic byLouis LevyProductioncompanyGainsborough PicturesDistributed byGeneral Film Distributors (UK)Release date31 August 1940Running time75 minutesCountryUKLanguageEnglish Charley's (Big-Hearted) Aunt is a 1940 British com...

Some bubble tea for you! Great you could join us! Stinglehammer (talk) 13:22, 12 November 2019 (UTC)Reply[reply] A goat for you! A goat for you to say thank you for joining us! V.madden25 (talk) 13:26, 12 November 2019 (UTC)Reply[reply] Welcome Hello, Cardillac2019, and Welcome to Wikipedia! Welcome to Wikipedia! I hope you enjoy the encyclopedia and want to stay. As a first step, you may wish to read the Introduction. If you have any questions, feel free to ask me at my talk page – I...

背泳ぎ 背泳ぎ(せおよぎ、英: backstroke)は、水泳で、仰向けの姿勢で泳ぐ泳ぎ方である。音読みで「背泳」(はいえい)とも呼ばれるが、競技規則では背泳ぎである。 水泳選手を中心に「バック」という略称が用いられている。 歴史 背泳ぎが行われるようになった時期は明らかでない[1]。古代エジプトのラムセス2世の葬祭殿のレリーフにあるカディシュの戦...
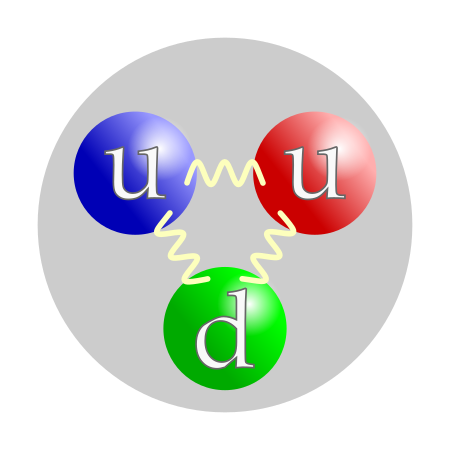
Particle smaller than an atom A composite particle proton is made of two up quark and one down quark, which are elementary particles. In physics, a subatomic particle is a particle smaller than an atom.[1] According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles (for example, a baryon, like a proton or a neutron, composed of three quarks; or a meson, composed of two quarks), or an elementary part...

Motor vehicle Iveco TurboCityAn Iveco 480 TurboCity in BucharestOverviewManufacturerIveco-FiatBody and chassisDoors2-4Floor typestep entrance, 0.735 m (2.4 ft) floor heightChassissemi-integralPowertrainEngineDieselCapacity105 / 115 passengersPower output159 kW, 200 kWTransmissionZF or VoithDimensionsWheelbase5.11 m (16.8 ft)6.15 m (20.2 ft)Length9.5 m (31.2 ft)10.7 m (35.1 ft)12 m (39.4 ft)17.8 m (58.4 ft)Width2.5...

2018 Thai television series YouniverseAlso known asYOUniverseThaiYOUniverse – จักรวาลเธอ GenreRomanceCreated byGMMTVDirected byPawis SowsrionStarringKorapat KirdpanPloyshompoo SupasapWachirawit RuangwiwatApichaya SaejungCountry of originThailandOriginal languageThaiNo. of episodes4ProductionRunning time8 – 11 minutesProduction companyGMMTVOriginal releaseNetworkLINE TVFacebookYouTubeRelease24 April (2018-04-24) –3 May 2018 (2018-05-03) Youniverse (styl...

This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Flag of Iron – news · newspapers · books · scholar · JSTOR (January 2011) (Learn how and when to remove this template message) 0000 Hong Kong filmThe Flag of IronDirected byChang ChehProduced byRun Run ShawStarring Kuo Chui Chiang Sheng Wang Li Lu Feng Lung Tung Sheng Distribute...

American TV series or program Tool AcademyStarringJordan MurphyTrina DolenzCountry of originUnited StatesNo. of seasons3No. of episodes27ProductionExecutive producersSallyAnn SalsanoJim AckermanDave HamiltonRunning time60 minutesProduction companies495 ProductionsVH1Original releaseNetworkVH1ReleaseJanuary 11, 2009 (2009-01-11) –April 4, 2010 (2010-04-04) Tool Academy is a competitive reality television show featuring unsuspecting bad boys (and women) who have been sent ...

Unincorporated community in Virginia, United States This article is about the community in Virginia. For the U.S. government communications facility, see Warrenton Training Center. Census-designated place in Virginia, United StatesBrandy Station, VirginiaCensus-designated placePortion of the battlefield in April 2017Location of the Brandy Station CDP within the Culpeper CountyBrandy StationLocation within the state of VirginiaShow map of VirginiaBrandy StationBrandy Station (the United States...

本表是動態列表,可能永遠無法完結。歡迎您參考可靠來源來查漏補缺。 擔任2003年和2007年天使隊開幕戰先發投手的約翰·雷基。 洛杉磯天使是位於美國加利福尼亞州安那罕的一支棒球隊,隸屬於美國職棒大聯盟美聯西區。天使隊於1961年成立,歷史上曾用過「洛杉磯天使」、「加州天使」、「洛杉磯安那罕天使」等隊名。[1]在新球季能夠擔任開幕戰的先發,對於先發�...

As referências deste artigo necessitam de formatação. Por favor, utilize fontes apropriadas contendo título, autor e data para que o verbete permaneça verificável. (Julho de 2022) Esta página cita fontes, mas que não cobrem todo o conteúdo. Ajude a inserir referências. Conteúdo não verificável pode ser removido.—Encontre fontes: ABW • CAPES • Google (N • L • A) (Dezembro de 2021) Trairiense Nome Associação Trairi...

يفتقر محتوى هذه المقالة إلى الاستشهاد بمصادر. فضلاً، ساهم في تطوير هذه المقالة من خلال إضافة مصادر موثوق بها. أي معلومات غير موثقة يمكن التشكيك بها وإزالتها. (سبتمبر 2023) هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها...

今池停留場 到着する列車と構内踏切と駅出入口階段 いまいけ IMAIKE ◄HN52 新今宮駅前 (0.4 km) (0.3 km) 今船 HN54► 所在地 大阪市西成区萩之茶屋2丁目2-7北緯34度38分43.97秒 東経135度30分7.86秒 / 北緯34.6455472度 東経135.5021833度 / 34.6455472; 135.5021833座標: 北緯34度38分43.97秒 東経135度30分7.86秒 / 北緯34.6455472度 東経135.5021833度 / 34.6455472; ...

Bukit Begoꦥꦸꦤ꧀ꦛꦸꦏ꧀ꦧꦺꦒꦺꦴJenisDestinasi wisata alamLokasiJalan Imogiri–Dlingo, Dusun Kedung Buweng, Kalurahan Wukirsari, Kapanéwon Imogiri, Kabupaten Bantul, Daerah Istimewa YogyakartaKoordinat7°55′33″S 110°24′12″E / 7.925729°S 110.403209°E / -7.925729; 110.403209Dibuka 2010 (perkiraan) 2019 (mulai populer) Dioperasikan olehDinas Pariwisata Kabupaten BantulStatusDibuka (sepanjang hari)Fasilitas Area parkir kendaraan Area moto...





