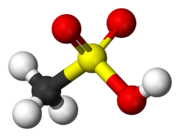
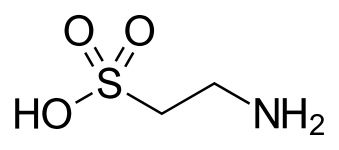
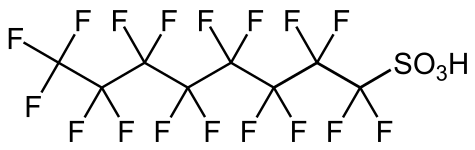
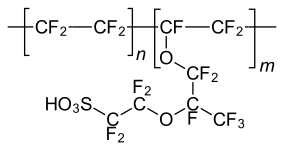
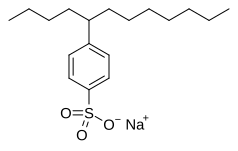
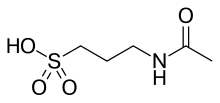
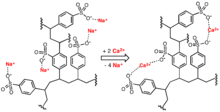
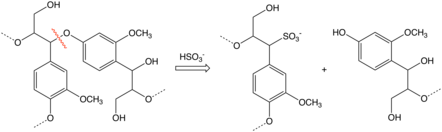
Sulfonic acid
|
Read other articles:

Beberapa atau seluruh referensi dari artikel ini mungkin tidak dapat dipercaya kebenarannya. Bantulah dengan memberikan referensi yang lebih baik atau dengan memeriksa apakah referensi telah memenuhi syarat sebagai referensi tepercaya. Referensi yang tidak benar dapat dihapus sewaktu-waktu. Mak Lampir, dengan nama sebenarnya adalah Siti Lampir Maimunah merupakan legenda yang berasal dari Sumatera Barat. Ada beberapa kisah dari legenda Mak Lampir yang beredar di tengah masyarakat Indonesia. Aw...
Den här artikeln har skapats av Lsjbot, ett program (en robot) för automatisk redigering. (2016-11)Artikeln kan innehålla fakta- eller språkfel, eller ett märkligt urval av fakta, källor eller bilder. Mallen kan avlägsnas efter en kontroll av innehållet (vidare information) Barrio de México Ort Land Mexiko Delstat Mexiko Kommun Zinacantepec Höjdläge 2 979 m ö.h. Koordinater 19°16′57″N 99°49′42″V / 19.2825°N 99.82833°V / 19.2...

Disambiguazione – Se stai cercando altri significati, vedi Cappadocia (disambigua). CappadociaKapadokya Panorama della regione Stati Turchia (Distretto dell'Anatolia Orientale) TerritorioTurchia interna CapoluogoKayseri, Nevşehir Nome abitanticappadoci Cartina fisica dell'Anatolia ellenistica Coordinate: 38°39′30″N 34°51′13″E / 38.658333°N 34.853611°E38.658333; 34.853611 La Cappadocia (AFI: /kappaˈdɔʧa/;[1] in turco Kapadokya dal persiano ant...

Die Chauken (Aussprache [çaʊkən], lateinisch: Chauci, griechisch: οἱ Καῦχοι; „die Hohen“) waren ein germanischer Stamm, der beidseits der unteren Weser (westlich: lat. chauci minores, östlich: lat. chauci maiores) lebte. Die Chauken gehörten nach Tacitus zur Gruppe der von der Nordseeküste stammenden Ingaevonen. Obwohl die frühere Forschung die Chauken durchaus in den Sachsen fortgesetzt sah, wird der Stamm heutzutage vermehrt mit der Genese der Franken in Zusammenhang ge...

Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. Cet article ne cite pas suffisamment ses sources (novembre 2014). Si vous disposez d'ouvrages ou d'articles de référence ou si vous connaissez des sites web de qualité traitant du thème abordé ici, merci de compléter l'article en donnant les références utiles à sa vérifiabilité et en les liant à la section « Notes et références » En pratique : Quelles sources sont attendues ? C...

село Пеулештій-НойPăuleștii Noi Країна Румунія Повіт Прахова Комуна Пеулешть Код SIRUTA 130883 Поштові індекси 107403 Телефонний код +40 244 (Romtelecom, TR)+40 344 (інші оператори) Координати 44°59′31″ пн. ш. 25°58′48″ сх. д.H G O Висота 202 м.н.р.м. Населення 350 (2002) Розташування Пеулештій-Н�...

The JucklinsIklanSutradara George Melford Produser Jesse L. Lasky Ditulis oleh Frank Condon SkenarioFrank CondonBerdasarkanThe Jucklinsoleh Opie ReadPemeranWinter HallMabel Julienne ScottMonte BlueRuth RenickFanny MidgleyZ. Wall CovingtonJ.M. DumontSinematograferPaul P. PerryPerusahaanproduksiFamous Players-Lasky CorporationDistributorParamount PicturesTanggal rilis 9 Januari 1921 (1921-01-09) Durasi50 menitNegara Amerika Serikat BahasaFilm bisu dengan antar judul Inggris The Jucklins ad...

Фредерік Наттер ЧейзенНародився 1896[1][2]Саффолк, Англія, Сполучене КоролівствоПомер 13 лютого 1942(1942-02-13)[1]СінгапурКраїна Велика БританіяДіяльність зоолог, орнітолог, ботанікПосада директор музеюdДіти Гізер Чейзенd Систематик живої природи Дослідник, як

Pakistan Army branch charged with the supply of weapons and ammunition Pakistan Army Corps of OrdnanceBadge of the Pakistan Army Ordnance CorpsActive1947; 76 years ago (1947)Country PakistanBranch Pakistan ArmyTypeCombat service supportRoleAdministrative and staffing oversight.SizeVariesHQ/GarrisonOrdnance Center in Malir Cantonment, Sindh, PakistanNickname(s)ORDAnniversaries1947EngagementsMilitary history of PakistanDecorationsMilitary Decorations of Pakistan mili...

Provost Ross's house in Shiprow, 2005 Shiprow is a historic street in the heart of Aberdeen, Scotland, near the harbour. Formerly the Shiprow sloped upward more gradually than it does now, and it crossed Union Street in a depression between St Catherine's Hill on the west and Castle Street, once high uneven ground, on the east. That the Shiprow has been made up several feet can be seen by a house at the end of Exchequer Row, and it crossed Union Street and entered Broad Street at a lower leve...

2006 single by Bob EvansDon't You Think It's Time?Single by Bob Evansfrom the album Suburban Songbook B-sideSisters Wedding DayReleased13 May 2006Recorded2005–2006GenreFolk popLength9:38LabelEMI AustraliaCapitol RecordsSongwriter(s)Bob EvansProducer(s)Bob Evans & Brad JonesBob Evans singles chronology Turn (2003) Don't You Think It's Time? (2006) Nowhere Without You (2006) Don't You Think It's Time? is the first single to be taken from Bob Evans' (aka Kevin Mitchell from Jebediah) secon...

National identity card of Norway Norwegian identity cardID-kortID-duođaštusFront of the cardBack of the cardTypeIdentity card,optional replacement for passport for travel to EU and EFTA countries.Issued by NorwayNorwegian Police ServiceFirst issued30 November 2020 (first version)29 July 2021 (current version)PurposeIdentification & travelValid inThe Nordic countries Norway Denmark Faroe Islands Finland Greenland[1] Iceland Swed...

AfriMusic Song Contest Programa de televisiónTambién conocido como Concours AfriMusic de la ChansonTítulos en español Festival de la Canción de AfriMusicCreado por Michelle FernandesSuzie VicenteVictor NunesBasado en Festival de la Canción de EurovisiónPaís de origen Lista de paísesN.º de temporadas 3 (hasta 2020 inclusive)ProducciónLugar(es) de producción ÁfricaProducciones relacionadas Festival de la Canción de EurovisiónEnlaces externos Sitio web oficial[editar datos en...

Los Angeles ClippersDatos generalesDeporte BaloncestoFundación 1970Historia Buffalo Braves 1970-1978 San Diego Clippers 1978-1984 Los Angeles Clippers 1984-PresenteColores Rojo, Azul, Negro, Gris y Blanco Propietario(s) Steve BallmerPresidente Lawrence FrankMánager general Trent ReddenEntrenador Tyronn LueEquipo afiliado Agua Caliente ClippersPatrocinador HoneyInstalac...

Human settlement in WalesMynythoLooking towards the southMynythoLocation within GwyneddPopulation536 OS grid referenceSH307311• Cardiff110 mi (177 km)CommunityLlanenganPrincipal areaGwyneddPreserved countyGwyneddCountryWalesSovereign stateUnited KingdomPost townPWLLHELIPostcode districtLL53Dialling code01758PoliceNorth WalesFireNorth WalesAmbulanceWelsh UK ParliamentDwyfor MeirionnyddSenedd Cymru – Welsh ParliamentDwyfor Meirionn...

Salah satu dari naskah-naskah Elefantin, memohon izin pembangunan rumah ibadah Yahudi di Elefantin. Naskah-naskah Elefantin adalah kumpulan naskah berupa akta, kontrak, dan surat yang ditemukan di Elefantin pada akhir abad ke-5.[1] Elefantin merupakan tempat pemukiman bangsa Yahudi yang ada di satu pulau kecil di Sungai Nil.[1] Letaknya di perbatasan sebelah selatan Mesir.[1] Naskah-naskah ini dituliskan di atas papirus dengan memakai bahasa Aram.[1][2]...

Geographical region Satellite imagery of the Southern Levant The Southern Levant is a geographical region encompassing the southern half of the Levant. It corresponds approximately to modern-day Israel, Palestine, and Jordan; some definitions also include southern Lebanon, southern Syria and/or the Sinai Peninsula. As a strictly geographical description, it is sometimes used by archaeologists and historians to avoid the religious and political connotations of other names for the area. Like mu...

Dan Steele Nazionalità Stati Uniti Altezza 188 cm Peso 100 kg Bob Specialità Bob a quattro Palmarès Competizione Ori Argenti Bronzi Giochi olimpici 0 0 1 Per maggiori dettagli vedi qui Atletica leggera Specialità Prove multiple Record Decathlon 8 130 p. (1999) Carriera Nazionale 1999 Stati Uniti Palmarès Competizione Ori Argenti Bronzi Giochi panamericani 0 1 0 Per maggiori dettagli vedi qui Modifica dati su Wikidata · Manuale Daniel Steele, detto Dan (Moline, 20 ...

Major League Baseball season Major League Baseball team season 1988 Boston Red SoxAmerican League East ChampionsLeagueAmerican LeagueDivisionEastBallparkFenway ParkCityBoston, MassachusettsRecord89–73 (.549)Divisional place1stOwnersJean Yawkey, Haywood SullivanPresidentJohn Harrington[a]General managerLou GormanManagersJohn McNamara (43–42)Joe Morgan (46–31)TelevisionWSBK-TV, Ch. 38(Sean McDonough, Bob Montgomery)NESN(Ned Martin, Jerry Remy)RadioWPLM-FM 99.1WPLM-AM 1390(Ken...

Lucy McRaeLucy McRae in 2012Born1979London, EnglandEducationRMITKnown forSci Fi art, film, biotech, art direction, edible, wearable tech Lucy McRae (born 1979) is a British-born Australian science fiction artist, body architect, film maker and TED fellow. Her installations include film, photography, sculptures, and edible and wearable technology. Career McRae uses technology and design to create what she calls body architecture. She describes herself artistically as exploring the limits...