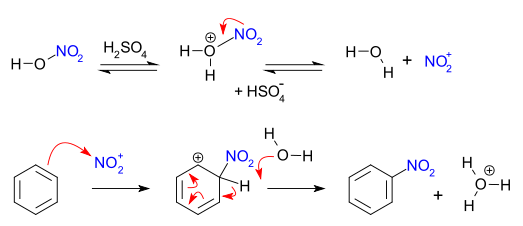
Nitration
|
Read other articles:

Mus√©e Mikha√Øl-BoulgakovLe mus√©e Mikha√Øl-Boulgakov, au no 13 de la descente Saint-Andr√©, √Ý Kiev.Informations g√©n√©ralesType B√¢timent, structure architecturale (en), monument du patrimoine architectural (d)Ouverture 15 mai 1991Site web www.bulgakov.org.uaB√¢timentProtection Monument du patrimoine architectural (d)LocalisationAdresse Descente Saint-Andr√© Kiev UkraineCoordonn√©es 50¬∞ 27‚Ä≤ 39‚Ä≥ N, 30¬∞ 30‚Ä≤ 53‚Ä≥ Emodifier - modifier le code - m...

Das Olympiazentrum Schilksee (S√ºdteil) ‚Äì im Hintergrund die beiden Apartmenth√§user Grundsteinlegung des Olympiazentrums Schilksee am 13. Oktober 1969 Das Olympiazentrum Schilksee (Nordteil) ‚Äì im Hintergrund das Hotel Olympia Die Fackel des olympischen Feuers auf der Aussichtsplattform der Hafenmeisterei Das Olympiazentrum Schilksee (auch Olympiazentrum Kiel-Schilksee ‚Äì gelegentlich auch (unzutreffend) als Olympiahafen Schilksee bezeichnet) ist ein Komplex aus Yachthafen, Wohngeb√...

British politician Doveridge Hall, Derbyshire Sir Henry Cavendish, 1st Baronet (13 April 1707 – 31 May 1776), was a British politician who held several appointments in the Kingdom of Ireland. Biography Cavendish was the son of William Cavendish and Mary Tyrell. He was descended from Sir William Cavendish, an ancestor shared with the dukes of Devonshire.[1] Cavendish studied at University College, Oxford, matriculating on 17 August 1724. He held the office of High Sheriff of Derbyshi...

–ë–æ–π–æ–≤–µ –∫–æ–º–∞–Ω–¥—É–≤–∞–Ω–Ω—è –ü–æ–≤—ñ—Ç—Ä—è–Ω–∏—Ö —Å–∏–ª –°–®–ê Air Combat Command –®—Ç–∞–± –ë–æ–π–æ–≤–æ–≥–æ –∫–æ–º–∞–Ω–¥—É–≤–∞–Ω–Ω—è –ü–æ–≤—ñ—Ç—Ä—è–Ω–∏—Ö —Å–∏–ª –°–®–ê–ù–∞ —Å–ª—É–∂–±—ñ 1 —á–µ—Ä–≤–Ω—è 1992 ‚Äî –ø–æ —Ç.—á.–ö—Ä–∞—ó–Ω–∞ –°–®–ê–í–∏–¥ –ü–° –°–®–ê–¢–∏–ø –ì–æ–ª–æ–≤–Ω–µ –∫–æ–º–∞–Ω–¥—É–≤–∞–Ω–Ω—è –ü–° –°–®–ê–Ý–æ–ª—å –ö–æ–º–∞–Ω–¥—É–≤–∞–Ω–Ω—è–í—ñ–π—Å—å–∫–æ–≤–æ-–ø–æ–≤—ñ—Ç—Ä—è–Ω–∞ –±–∞–∑–∞ –õ–µ–Ω–≥–ª—ñ, –õ–µ–Ω–≥–ª—ñ-–Æ—Å—Ç—ñ—Å, –î–∂–æ—Ä–¥–∂—ñ—è–ü—Ä—ñ–∑–≤–∏—Å—å–∫–∞ ACC–ì...

Football team of La Gauloise de Trinité For the parent multi-sport club, see La Gauloise de Trinité. Football clubLa Gauloise de TrinitéFounded1920StadiumStade Louis Richer[1]Capacity2,000[2]LeagueRégional 2 MartiniqueGroup B[3] La Gauloise de Trinité is a football team located in La Trinité, Martinique. It is part of the multi-sport club La Gauloise de Trinité. History The club of La Gauloise de Trinité was founded in 1920, and so was its football team.[4&...

1996 film Thieves(Les Voleurs)Theatrical release posterDirected byAndré TéchinéWritten byAndré Téchiné Gilles Taurand Michel AlexandreProduced byAlain SardeStarringDaniel AuteuilCatherine Deneuve Laurence CôteCinematographyJeanne LapoirieEdited byMartine GiordanoMusic byPhilippe SardeRelease dates May 1996 (1996-05) (Cannes) 21 August 1996 (1996-08-21) (France) Running time117 minutesCountryFranceLanguageFrenchBudget8.400.000 €[1]Box office$17...

British TV series or programme PiratesGenreChildren'sComedyWritten byRebecca StevensPeter TabernMatthew TabernDave FreedmanAlan GilbeyMichael MalaghanDirected byPeter TabernStarringLiz SmithPaul BownAndrew SachsHayley ElliottBenjamin RennisRebecca StevensToby SedgwickTheme music composerJonathan CohenComposerJonathan CohenCountry of originUnited KingdomOriginal languageEnglishNo. of series3No. of episodes24ProductionExecutive producersAlbert BarberJeremy SwanProducerPeter TabernRunning t...

–¶—è —Å—Ç–∞—Ç—Ç—è –Ω–µ –º—ñ—Å—Ç–∏—Ç—å –ø–æ—Å–∏–ª–∞–Ω—å –Ω–∞ –¥–∂–µ—Ä–µ–ª–∞. –í–∏ –º–æ–∂–µ—Ç–µ –¥–æ–ø–æ–º–æ–≥—Ç–∏ –ø–æ–ª—ñ–ø—à–∏—Ç–∏ —Ü—é —Å—Ç–∞—Ç—Ç—é, –¥–æ–¥–∞–≤—à–∏ –ø–æ—Å–∏–ª–∞–Ω–Ω—è –Ω–∞ –Ω–∞–¥—ñ–π–Ω—ñ (–∞–≤—Ç–æ—Ä–∏—Ç–µ—Ç–Ω—ñ) –¥–∂–µ—Ä–µ–ª–∞. –ú–∞—Ç–µ—Ä—ñ–∞–ª –±–µ–∑ –¥–∂–µ—Ä–µ–ª –º–æ–∂–µ –±—É—Ç–∏ –ø—ñ–¥–¥–∞–Ω–æ —Å—É–º–Ω—ñ–≤—É —Ç–∞ –≤–∏–ª—É—á–µ–Ω–æ. (28 —Å–µ—Ä–ø–Ω—è 2021) ¬´–®–∞–ª–µ–Ω–∞ —Ñ—ñ–≥—É—Ä–∞¬ª ‚Äî –Ω–µ–∑–∞—Ö–∏—â–µ–Ω–∞ —Ñ—ñ–≥—É—Ä–∞, —è–∫–∞ –Ω–∞–ø–∞–¥–∞—î –Ω–∞ —Ñ—ñ–≥—É—Ä—É —Å—É–ø–...

Tata IslandsTata Islands seen from Tata BeachGeographyLocationGolden Bay, New ZealandCoordinates40°48′07″S 172°54′40″E / 40.802°S 172.911°E / -40.802; 172.911Adjacent toGolden BayTotal islands2Major islandsMotu Island Ngawhiti IslandArea4.6 ha (11 acres)Highest elevation32 m (105 ft)AdministrationNew ZealandDistrictTasman Tata Islands are a pair of small uninhabited islands off the north coast of New Zealand's South Island. They are loca...

1962 film This article is missing information about the film's production, and theatrical release. Please expand the article to include this information. Further details may exist on the talk page. (February 2018) El Baron del TerrorMexican theatrical release posterDirected byChano UruetaScreenplay byFederico CurielAdolfo López PortilloStory byFederico CurielAdolfo López PortilloProduced byAbel SalazarStarringAbel SalazarCinematographyJosé Ortiz RamosEdited byAlfredo Rosas PriegoMusic byGu...

This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Mystic Towers – news · newspapers · books · scholar · JSTOR (January 2019) (Learn how and when to remove this template message) 1994 video gameMystic TowersDeveloper(s)Animation F/XPublisher(s)AU: ManaccomWW: Apogee SoftwareDesigner(s)Lindsay WhippPlatform(s)MS-DOS, Windows, Mac...

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Bulu Cina, Hamparan Perak, Deli Serdang – berita · surat kabar · buku · cendekiawan · JSTOR Bulu CinaDesaNegara IndonesiaProvinsiSumatera UtaraKabupatenDeli SerdangKecamatanHamparan PerakKode pos203...

Chalcedon FoundationFounded1965 (1965)FounderRousas John RushdoonyTypeNonprofit 501(c)(3)Tax ID no. 95-6121940 (EIN)LocationVallecito, CaliforniaMembers 3OwnerChalcedon, Inc.Key peopleMark R. Rushdoony, PresidentMartin G. Selbrede, Vice PresidentRevenue $961,294 (2010)[1]Employees 10Volunteers 10Websitechalcedon.edu This article is part of a series onConservatismin the United States Schools Compassionate Fiscal Fusion Libertarian Moderate Movement Neo Paleo Progressive Social Tra...

Square in Warsaw, Poland Warsaw Uprising Square; view to the north 52¬∞14‚Ä≤9‚Ä≥N 21¬∞00‚Ä≤47‚Ä≥E / 52.23583¬∞N 21.01306¬∞E / 52.23583; 21.01306 Warsaw Uprising Square (Polish: Plac Powsta≈Ñc√≥w Warszawy), still popularly known by its former name Napoleon Square (Polish: Plac Napoleona), is a square in the central Warsaw district of ≈ör√≥dmie≈õcie. Located at the junction of ulica ≈öwiƒôtokrzyska (Holy Cross Street) and ulica Szpitalna (Hospital Street) and near Nowy ≈...

Use of cannabis in Sweden Advertisement for cannabis from the 1800s Cannabis in Sweden is illegal for all purposes. It is illegal for recreational purposes, for most medical purposes and possession of even small amounts of cannabis is a criminal offence. Consequently, limited medical usage of cannabis-based drugs is only allowed for specific conditions. Attitudes Swedish singer Ängie has spoken about the attitudes towards cannabis in Sweden. In an interview in September 2016 she said, In Swe...

The Best of What's Around redirects here. For the compilation album, see The Best of What's Around Vol. 1. 1994 studio album by Dave Matthews BandUnder the Table and DreamingStudio album by Dave Matthews BandReleasedSeptember 27, 1994 (1994-09-27)RecordedMay 1994StudioBearsville (Woodstock, New York)GenreRockalternative rockjam rockLength62:52LabelRCAProducerSteve LillywhiteDave Matthews Band chronology Recently(1994) Under the Table and Dreaming(1994) Crash(1996) Singl...

Sibu Rural District Council Majlis Daerah Luar Bandar SibuLocal Authorities Ordinance 1996TypeTypeLocal authority of the Sibu Jaya and Selangau HistoryFounded1 January 1952LeadershipChairmanCr. Sempurai Petrus Ngelai Deputy ChairmanCr Wong Ching Yong (appoint on 1 August 2020) SecretaryMr Ng Siang Wei (January 2023) MottoT.E.A.M[1] (Together Everyone Achieve More)Meeting placeSibu Rural District Council, Floor 18, Wisma Sanyan, Sibu, Sarawak.Websitewww.srdc.gov.myFootnotesKnown as Day...

『オフィーリア』英語: Ophelia作者ジョン・エヴァレット・ミレー製作年1851年 - 1852年種類油彩、キャンバス寸法76.2 cm × 111.8 cm (30.0 in × 44.0 in)所蔵テート・ブリテン、ロンドン 『オフィーリア』(英: Ophelia)は、1851年から1852年にかけて制作されたジョン・エヴァレット・ミレーによる絵画である。 ロンドンにあるテート・ブリテン美術...

Questa voce sull'argomento centri abitati del Kentucky è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Waltoncity(EN) Walton, Kentucky Walton – Veduta LocalizzazioneStato Stati Uniti Stato federato Kentucky ConteaBooneKenton TerritorioCoordinate38°51′43.92″N 84°36′52.92″W38°51′43.92″N, 84°36′52.92″W (Walton) Altitudine283 m s.l.m. Superficie11,11 km² Acque i...

Public park in Staten Island, New York Pond Beach in the park Wolfes Pond Park is a large public park located on Staten Island's South Shore. It is bounded on the south by Holton Avenue, on the east by Raritan Bay, on the west by the Staten Island Railway, and on the north by Chisholm Street, Luten Avenue, and Cornelia Avenue, which is also the main entry into the park's public areas.[1] Hylan Boulevard bisects the park, and most visitors only visit the eastern half of the park, where...