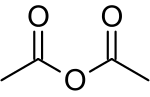
Acetic anhydride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Revolutionary SistersPoster promosiHangul오케이 광자매 Hanja오케이 光姊妹 GenreDrama keluargaPercintaanDrama komediCerita seruMisteriPembuatKi Min-soo Moon Jun-ha KBS Drama ProductionDitulis olehMoon Young-namSutradaraLee Jin-seoPemeranYoon Joo-sangHong Eun-heeJeon Hye-binKim Kyung-namGo Won-heeLee Bo-heeLee Byung-joonChoi Dae-chulPenata musikJang Young-kyu HaemiNegara asalKorea SelatanBahasa asliKoreaJmlh. episode50ProduksiProduser eksekutifKang Byeong-taek (KBS) Choi Hee-s...

أمبيلاكيا خريطة الموقع تقسيم إداري البلد اليونان [1] إحداثيات 37°57′00″N 23°31′00″E / 37.95°N 23.51666667°E / 37.95; 23.51666667 السكان التعداد السكاني 4710 (إحصاء السكان) (2011) معلومات أخرى التوقيت ت ع م+02:00 (توقيت قياسي)، وت ع م+03:00 (توقيت صيفي) الرمز البريدي 189 02&...

Đài Loan Tên bản ngữ 臺灣 (tiếng Quan thoại Đài Loan)[I]Tʻaiwan臺灣 (tiếng Nhật)Taiwan 1895–1945 Quốc kỳ Quốc huy Quốc ca: KimigayoQuân chi đạiẤn chương Tổng đốc phủ Đài Loan Đài Loan (đỏ) trong Đế quốc Nhật Bản (đỏ nhạt) ở mức độ xa nhất.Tổng quanVị thếThuộc địa của Đế quốc Nhật BảnThủ đôvà thành phố lớn nhất TaihokuNgô...

У Вікіпедії є статті про інших людей із прізвищем Конрад. Отто Конрад Отто Конрад Особисті дані Повне ім'я Отто Конрад Народження 1 листопада 1964(1964-11-01) (59 років) Ґрац, Австрія Зріст 187 см Громадянство Австрія Позиція воротар Юнацькі клуби «Grazer Sportklub» Професіональн...

Sports season2023 Archery World CupSportArcheryDuration18 April – 10 SeptemberWorld Cup FinalRecurve Men Marcus D'Almeida Lee Woo-seok Mauro NespoliRecurve Women Kang Chae-young Alejandra Valencia Lim Si-hyeonCompound Men Mathias Fullerton Prathamesh Samadhan Jawkar Mike SchloesserCompound Women Sara López Tanja Gellenthien Dafne QuinteroSeasons← 20222024 → The 2023 Archery World Cup, also known as the Hyundai Archery World Cup for sponsorship reasons, is the 17th edition of ...

A multidisciplinary peacekeeping force Map of the partitioning of RECs and RMs of the ASF The African Standby Force (ASF) (French: Force africaine en attente)[1] is an international, continental African, and multidisciplinary peacekeeping force with military, police and civilian contingents that acts under the direction of the African Union. The ASF is to be deployed in times of crisis in Africa.[2] Addis Ababa, Ethiopia, serves as the Force's Headquarters. Douala, Cameroon, w...

كريمة صالح جاسم كريمة جاسم في بطولة العالم لاختراق الضاحية 2013. معلومات شخصية الميلاد 18 فبراير 1988 (العمر 35 سنة) كينيا الجنسية البحرين الحياة العملية المهنة منافسة ألعاب القوى الرياضة 5000 متر، 10000 متر تعديل مصدري - تعديل سجل الميداليات منافس من البحرين ألعاب قو...

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (مارس 2023) هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (يناير 2016) أدب 1836معلومات عامةالسنة 1836 1835 في الأدب 1837 �...

Sports season2022 Big 12 Conference football seasonLeagueNCAA Division I FBS football seasonSportfootballDurationSeptember 3, 2022January 2023Number of teams10TV partner(s)Fox Family (Fox, FS1), ESPN Family (ABC, ESPN, ESPN2, ESPN3, ESPNU, Big 12 Now, LHN)2023 NFL DraftTop draft pickDE Tyree Wilson, Texas TechPicked byLas Vegas Raiders, 7th overallChampionship GameChampionsKansas State Runners-upTCUFinals MVPDeuce Vaughn, RBSeasons← 20212023 → 2022 Big 12 Conference f...

ملح هذا البحرملح هذا البحرملصق فيلم ملح هذا البحرمعلومات عامةالصنف الفني دراما- رومانسيتاريخ الصدور 2008 سبتمبر 3مدة العرض 109 دقيقةاللغة الأصلية اللغة العربيةالبلد فلسطينموقع الويب philistinefilms.org[1] الطاقمالمخرج آن ماري جاسرالكاتب آن ماري جاسرالسيناريو آن ماري جاسر البطول...

Anime convention in Boston Anime BostonStatusActiveVenueHynes Convention Center and Sheraton Boston HotelLocation(s)Boston, MassachusettsCountryUnited StatesInaugurated2003Attendance29,849 in 2022[1]Organized byNew England Anime Society[2]Websitewww.animeboston.com Anime Boston is an annual three-day anime fan convention held in the spring in Boston, Massachusetts, United States. Anime Boston was created and is run by the New England Anime Society, Inc., a Massachusetts-based ...

لمعانٍ أخرى، طالع جيمي كيلي (توضيح). هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (سبتمبر 2018) جيمي كيلي معلومات شخصية الميلاد 29 ديسمبر 1907(1907-12-29)سيهام [لغات أخرى] الوفاة 27 يوليو 1984 (عن عمر ناهز 76...

Search engine metrics company (founded 2008) Semrush Holdings, Inc.TypePublicTraded asNYSE: SEMRIndustrySearch engine marketingFounded2008; 15 years ago (2008)FoundersOleg ShchegolevDmitri MelnikovHeadquartersBoston, Massachusetts[1], United States of AmericaKey peopleOleg Shchegolev (CEO)Dmitri Melnikov (COO)Brian Mulroy (CFO)Eugene Levin (CSO)Revenue$254.3 million (2022)[2]Number of employees1,004 (2022)[3]Websitewww.semrush.com Semrush Holding...

عبد الرحمن البزاز القائم بأعمال رئيس الجمهورية العراقية في المنصب13 نيسان 1966 – 16 نيسان 1966 رئيس الوزراء نفسه عبد السلام عارف عبد الرحمن عارف رئيس وزراء العراق الحادي والخمسين رئيس الوزراء الخامس لللجمهورية العراقية في المنصب21 سبتمبر 1965 – 9 اغسطس 1966 الرئيس عبد السلام ع...

Stasiun Kampung Baru Kampung Baru Bekas stasiun Kampung baru yang kini menjadi warung atau rumah wargaLokasiKampung Baru, Medan Maimun, Medan, Sumatera UtaraIndonesiaKoordinat3°33′09″N 98°41′22″E / 3.5524814°N 98.6894163°E / 3.5524814; 98.6894163Koordinat: 3°33′09″N 98°41′22″E / 3.5524814°N 98.6894163°E / 3.5524814; 98.6894163OperatorKereta Api IndonesiaDivisi Regional I Sumatera Utara dan AcehLetak dari pangkal km 4+4467...

American college football season 2013 Arkansas–Pine Bluff Golden Lions footballConferenceSouthwestern Athletic ConferenceDivisionWest DivisionRecord2–9 (2–7 SWAC)Head coachMonte Coleman (6th season)Offensive coordinatorEric Dooley (2nd season)Defensive coordinatorMonte Coleman (8th season)Home stadiumGolden Lion Stadium(Capacity: 16,000)Seasons← 20122014 → 2013 Southwestern Athletic Conference football standings vte Conf Overall Team W &#...

Nebula surrounding a dying star Not to be confused with Protoplanetary disk. The Westbrook Nebula, a protoplanetary nebula. A protoplanetary nebula or preplanetary nebula (Sahai, Sánchez Contreras & Morris 2005) (PPN, plural PPNe) is an astronomical object which is at the short-lived episode during a star's rapid evolution between the late asymptotic giant branch (LAGB)[a] phase and the subsequent planetary nebula (PN) phase. A PPN emits strongly in infrared radiation, and is a kind of r...

People of Andaman archipelago Andamanese redirects here. For other uses, see Andamanese (disambiguation). AndamaneseMembers of an unspecified Andamanese tribe fishing in c. 1870Total populationc. over 500Regions with significant populations India Andaman IslandsLanguagesGreat Andamanese languagesOngan languages (Onge, Jarawa)Sentinelese[note 1]ReligionAndamanese animism The Andamanese are the various indigenous peoples of the Andaman Islands, part of India's Andaman a...

Public community college in Winsted, CT Northwestern Connecticut Community CollegeMottoThe small college that does great thingsTypePublic community collegeEstablished1965Parent institutionConnecticut State Colleges & UniversitiesAcademic affiliationSpace-grantPresidentMichael Rooke, Ph.DUndergraduates1600LocationWinsted, Connecticut, United StatesCampusRuralWebsitewww.nwcc.commnet.edu Northwestern Connecticut Community College (NCCC) is a public community college in Winsted, Connecticut. ...

ウラジーミル・キリロヴィチВлади́мир Кири́ллович ホルシュタイン=ゴットルプ=ロマノフ家 ウラジーミル・キリロヴィチ称号 ロシア大公出生 (1917-08-30) 1917年8月30日 フィンランド大公国、ウーシマー州ポルヴォー死去 (1992-04-21) 1992年4月21日(74歳没) アメリカ合衆国、フロリダ州マイアミ埋葬 ロシア、サンクトペテルブルク、ペトロパヴロフスク要塞内首座�...