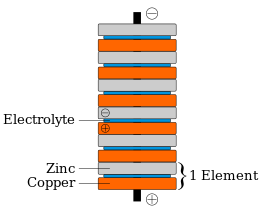
Voltaic pile
|
Read other articles:

Menara Syahbandar (Uitkijk) dibangun sekitar tahun 1839 yang berfungsi sebagai menara pemantau bagi kapal-kapal yang keluar-masuk Kota Batavia lewat jalur laut serta berfungsi kantor pabean yakni mengumpulkan pajak atas barang-barang yang dibongkar di pelabuhan Sunda Kelapa. Menara ini sebenarnya menempati bekas bastion (selekoh) Culemborg yang dibangun sekitar 1645, seiring pembuatan tembok keliling kota di tepi barat. Sebelum dibangun Menara Syahbandar, fungsi menara pemantau sudah dibangun di…
У Вікіпедії є статті про інших людей із прізвищем Ковальов. Альберт Ковальов Особисті дані Повне ім'я Ковальов Альберт Сергійович Народження 4 березня 1971(1971-03-04) (52 роки) Жданов, СРСР Зріст 179 см Вага 74 кг Громадянство СРСР → Україна Позиція захисник / півзахисни

Хрест заслуги Буковинського Куреня — пам'ятна відзнака для учасників бойових дій з участю Буковинського Куреня, а також інших осіб, причетних до діяльності куреня Лицева сторона Хреста заслуги Буковинського Куреня Пам'ятник Героям Буковинського куреня (Чернівці, 1995) З…

Pour les articles homonymes, voir Ancien hôpital. Ancien hôpital de MontbéliardAncien hôpital de MontbéliardPrésentationType Structure médicaleStyle XVIIIe siècleArchitecte Jean Frédéric FALLOTConstruction 1762Propriétaire Ville de Montbéliard (d)Patrimonialité Inscrit MH (1989)LocalisationPays FranceRégion Bourgogne-Franche-ComtéCommune MontbéliardAdresse 1, rue du ChâteauAccès et transportAutobus évolitY Lignes 1 2 3 B C D̴…

Football clubAldosiviFull nameClub Atlético AldosiviNickname(s)Tiburón (Shark)El Verde (The Green)El equipo de la Ciudad (The City team)El más grande de la Costa Atlantica (The greatest on the Atlantic Coast)Founded29 March 1913; 110 years ago (1913-03-29)GroundEstadio José María Minella,Mar Del Plata, Buenos Aires ProvinceCapacity35,354 [1]ChairmanJosé MoscuzzaManagerLeandro SomozaLeaguePrimera Nacional2023Primera Nacional Zone B, 16thWebsiteClub website Home col…

Steve CarellCarell pada Acara Perdana Crazy, Stupid, Love. di Sydney, Juli 2011LahirSteven John Carell16 Agustus 1962 (umur 61)Concord, Massachusetts, Amerika SerikatAlmamaterDenison UniversityPekerjaanAktor, pelawak, artis suara, produser, penulis, sutradaraTahun aktif1989–kiniKarya terkenalThe Daily Show The OfficeSuami/istriNancy Carell (m. 1995–kini)Anak2PenghargaanGolden Globe Award for Best Actor in a Television Comedy Series2006 The Office Steven John Carrell (lahir 16 Agust…

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (يناير 2018) تعتبر سلطة الراهب خفيفة وسهلة التحضير، وتعتبر من أطباق المطبخ اللبناني. تحضّر سلطة الراهب من الباذنجان، الطماطم، البصل، البقدونس، عصير الليمون الحامض، الزيت…

Professional boxing match Final JudgmentDateSeptember 15, 2018VenueT-Mobile Arena, Paradise, Nevada, U.S.Title(s) on the lineWBA (Super), WBC, IBO, and vacant The Ring middleweight titlesTale of the tapeBoxer Canelo Álvarez Gennady GolovkinNickname Canelo(Cinnamon) GGGHometown Guadalajara, Jalisco, Mexico Karagandy, KazakhstanPre-fight record 49–1–2 (34 KO) 38–0–1 (34 KO)Height 5 ft 8 in (173 cm) 5 ft 10+1⁄2 in (179 cm)Weight 159+2⁄5 lb (72…

زين العطار معلومات شخصية مكان الميلاد شيراز تاريخ الوفاة سنة 1403 مواطنة إيران الحياة العملية المهنة طبيب تعديل مصدري - تعديل علي بن الحسن زين الدين الأنصاري وشهرته الحاج زين العطار (ت. 806 هـ[1]) هو طبيب فارسي. من آثاره: «اختيارات البديعي» ألفه في سنة 770 هـ.[2] …

Business Motivation Model The Business Motivation Model (BMM) in enterprise architecture provides a scheme and structure for developing, communicating, and managing business plans in an organized manner.[1] Specifically, the Business Motivation Model does all the following: identifies factors that motivate the establishing of business plans; identifies and defines the elements of business plans; and indicates how all these factors and elements inter-relate. History Initially developed by…

Homer Davenport 1907 Homer Calvin Davenport (* 8. März 1867 in den Waldo Hills bei Silverton, Oregon; † 2. Mai 1912) war ein US-amerikanischer Cartoonist. Seine Zusammenarbeit mit William Randolph Hearst machte ihn zum einflussreichsten politischen Karikaturisten der USA in seiner Zeit. Seine Hauptfigur war Davvy. Davenport trug maßgeblich zur Wahl des Präsidentschaftskandidaten Theodore Roosevelt im Jahr 1904 bei. Inhaltsverzeichnis 1 Leben 2 Davenport als Pferdezüchter 3 Eheprobleme und …

Class of transport proteins Acid-sensing sodium channelStructure of acid-sensing ion channel 1.[1]IdentifiersSymbolASCPfamPF00858InterProIPR001873PROSITEPDOC00926SCOP22qts / SCOPe / SUPFAMTCDB1.A.6OPM superfamily181OPM protein4fz1Available protein structures:Pfam structures / ECOD PDBRCSB PDB; PDBe; PDBjPDBsumstructure summary Acid-sensing ion channels (ASICs) are neuronal voltage-insensitive sodium channels activated by extracellular protons permeable to Na+. ASIC1 also show…

Historia Caroli MagniOtros nombres Pseudo-Turpin Historia Karoli Magni y Rotholandi Crónica de Carlomagno y Roldán Historia TurpiniAutor Turpín (atribución no concluyente)Fecha siglo XI-XIIIdioma francés[editar datos en Wikidata] La Historia Caroli Magni, o Crónica de Turpin o el Pseudo-Turpin, o Crónica de Carlomagno y Roldán (en latín Historia Karoli Magni y Rotholandi), es una obra del siglo XI-XII escrita en francés cuyo autor es desconocido, presentándose como…

此條目介紹的是其他以英格瑞或英格麗德命名熱帶氣旋。关于其他用法,请见「熱帶氣旋英格瑞」。 超强台风英格丽德Typhoon Ingrid(英文)1946年7月18日的天氣圖(由日本氣象廳繪畫)路徑圖超强台风英格丽德的路徑圖十分鐘平均風速颱風(JMA)175 km/h(95 kt)強颱風 (HKO)175 km/h二分鐘平均風速强台风 (CMA)175 km/h(48 m/s)一分鐘平均風速颱�…

Railway station in Kepong, Malaysia KA06 Kepong Commuter rail stationGeneral informationOther namesChinese: 甲洞LocationKepong, Kuala Lumpur, Malaysia.Owned byKeretapi Tanah MelayuLine(s)2 Tanjung Malim-Port Klang Line (KTM Komuter) (2015 to present)Platforms2 side platformTracks4ConstructionParkingAvailableOther informationStation code KA06 HistoryOpened1892Rebuilt1995Electrified1995Services Preceding station Keretapi Tanah Melayu(Komuter) Following station Kepong Se…

English noblewoman Anne BeauchampDrawing of Anne from the Rous Roll, c. 1483Born14 February 1444 (1444-02-14)Cardiff, Glamorgan, WalesDied3 June 1449 (1449-06-04) (aged 5)Ewelme, Oxfordshire, EnglandResting placeReading Abbey, Berkshire51°27′22.85″N 0°57′54.31″W / 51.4563472°N 0.9650861°W / 51.4563472; -0.9650861Title15th Countess of Warwick7th Baroness BurghershTerm11 June 1446 – 3 June 1449SuccessorRichard NevilleParentsHenry Beauchamp, 1st …

Kepolisian Daerah BaliLambang Polda BaliSingkatanPolda BaliMottoSura Dwipa Sarva BavenaIkhtisarDibentuk1945Struktur yurisdiksiWilayah hukumProvinsi BaliMarkas besarJl. WR Supratman No.7, Sumerta Kauh, Kec. Denpasar Tim., Kota Denpasar, Bali 80236 Polisi Daerah Bali (dulu bernama Komando Daerah Polisi (Komda atau Koda)Bali) adalah pelaksana tugas Polisi Republik indonesia di wilayah Provinsi Bali. Polda Bali karena tergolong polda tipe A, dipimpin oleh seorang kepala kepolisian daerah yang berpan…

Steroidal antiandrogen and antimineralocorticoid Not to be confused with Spirolactone. SpironolactoneClinical dataPronunciation/ˌspaɪroʊnoʊˈlæktoʊn/ SPY-roh-noh-LAK-tone,[1] /ˌspɪəroʊnoʊˈlæktoʊn/ SPEER-oh-noh-LAK-tone[2] Trade namesAldactone, othersOther namesSC-9420; NSC-150339; 7α-Acetylthiospirolactone; 7α-Acetylthio-17α-hydroxy-3-oxopregn-4-ene-21-carboxylic acid γ-lactoneAHFS/Drugs.comMonographMedlinePlusa682627License data US DailyMed: Spir…

American sports television channel Television channel Next Level SportsCountryUnited StatesBroadcast areaNationwideProgrammingLanguage(s)EnglishOwnershipOwnerLax United Marketing, LLCHistoryLaunchedMarch 16, 2017 (2017-03-16)ReplacedOne World SportsFormer namesEleven Sports Network (2017–2021)LinksWebsitenlse.comAvailabilityStreaming mediaFuboTVpaid subscription serviceSamsung TV Plus1167 (FTF)Twitchtwitch.tv/FTFNextThe Roku Channel228 (FTF) Next Level Sports is an American …

Princess Takamado HisakoPrincess TakamadoPrincess Takamado in 2019BornHisako Tottori (鳥取久子) (1953-07-10) July 10, 1953 (age 70)Shirokane, Tokyo, JapanSpouse Norihito, Prince Takamado (m. 1984; died 2002)Issue Princess Tsuguko of Takamado Noriko Senge Ayako Moriya HouseImperial House of Japan (by marriage)FatherShigejiro TottoriMotherFumiko Tomoda Japanese imperial family The EmperorThe Empress Princess Toshi The Emperor EmeritusThe…