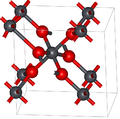
Lead dioxide
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Hereditary Grand Duchess of Oldenburg Princess ElisabethHereditary Grand Duchess of OldenburgBorn(1857-02-08)8 February 1857Potsdam, Kingdom of PrussiaDied28 August 1895(1895-08-28) (aged 38)Fulda, Prussia, German EmpireBurialDucal (Herzogliches) Mausoleum, Gertrudenfriedhof, OldenburgSpouse Frederick Augustus, Hereditary Grand Duke of Oldenburg (m. 1878)IssueSophia Charlotte, Princess Eitel Friedrich of PrussiaDuchess MargaretHouseHohenzollernFathe...

Bupati Kayong UtaraLambang Kabupaten Kayong UtaraPetahanaCitra Duanisejak 19 September 2018Masa jabatan5 tahunDibentuk2007Pejabat pertamaDrs. H. Syarif Umar AlkadrieSitus webkayongutarakab.go.id Berikut ini adalah Daftar Bupati Kayong Utara yang menjabat sejak pembentukannya pada tahun 2007. No Bupati Mulai menjabat Akhir menjabat Prd. Ket. Wakil Bupati — Drs. H. Syarif Umar Alkadrie 2007 2008 — [Ket. 1] — 1 H. Hildi Hamid 25 Juni 2008 25 Juni 2013 1 Ir. H. Muhammad Said Ti...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Real Thing Shakes – news · newspapers · books · scholar · JSTOR (May 2013) (Learn how and when to remove this template message) 1996 single by B'zReal Thing ShakesSingle by B'zReleasedMay 15, 1996GenreHard rockLabelBMG JapanSongwriter(s)Koshi Inaba, Tak Matsumo...

Зображення було скопійовано з wikipedia:en. Оригінальний опис містив: Summary Обґрунтування добропорядного використання для статті «Rebirth of a Nation» [?] Опис Обкладинка {{{Type}}} «Rebirth of a Nation», виконавець Public Enemy. Вважається, що авторське право на обкладинку належить лейблу, Gue...

Aeropuerto Internacional de la Capital IATA: CCE OACI: HECP FAA: LocalizaciónUbicación El Cairo, EgiptoSirve a Nueva Capital AdministrativaDetalles del aeropuertoTipo PúblicoPistas DirecciónLargoSuperficie01/193.650AsfaltoMapa CCE / HECP Ubicación en Egipto[editar datos en Wikidata] El Aeropuerto Internacional de la Capital (en árabe: مطار العاصمة الدولي) (IATA: CCE, OACI: HECP) es el principal aeropuerto internacional que sirve de contacto a la Nu...

Antonín Panenka Informasi pribadiNama lengkap Antonín PanenkaTanggal lahir 2 Desember 1948 (umur 75)Tempat lahir Praha, CekoslowakiaTinggi 1,78 m (5 ft 10 in)Posisi bermain GelandangKarier junior1958–1967 Bohemians PrahaKarier senior*Tahun Tim Tampil (Gol)1967–1981 Bohemians Praha 230 (76)1981–1985 Rapid Vienna 127 (63)1985–1987 VSE St. Pölten 1987–1989 SK Slovan Wien Total 357 (139)Tim nasional1973–1982 Cekoslowakia 59 (17) * Penampilan dan gol di klub seni...

Військова справатаВійна Історія Доісторична Антична Середньовічна Початок сучасної Індустріальна Сучасна Війна четвертого покоління Бойовий простір Повітря Кібер Інформація Суходіл Море Космос Зброя Бронетанкова Артилерія Кіннота Піхота Камуфляж Біологічна Геофі�...

1994 drama TV film Lakota Woman: Siege at Wounded KneeFilm posterGenreDramaBased onLakota Woman by Mary Crow DogWritten byBill KerbyRichard ErdoesDirected byFrank PiersonStarringIrene BedardMusic byRichard HorowitzCountry of originUnited StatesOriginal languagesEnglishLakotaProductionExecutive producersLois BonfiglioRobert M. SertnerFrank von ZerneckProducersFred BernerSteven P. SaetaAri Sloane (associate producer)CinematographyToyomichi KuritaChristopher TuftyEditorKatina ZinnerRunning time1...

طائرة عائمة دي هافيلاند توين أوتر تكمل هبوطها على الماء رحلة الخطوط الجوية الأمريكية رقم 1549 «حَطَّت على الماء» في نهر هدسون في عام 2009 وبقي جميع الركاب على قيد الحياة. في مجال الطيران، الهبوط على الماء (water landing)، يعتبر بالمعنى الواسع، هبوط طائرة على مسطح مائي. تهبط الطائرات ال

Pesawat terbang berbadan lebar Airbus A340-300 milik Virgin Atlantic Airways. Pesawat bebadan lebar terbesar kedua, Boeing 747 milik Garuda Indonesia Pesawat terbang berbadan lebar adalah pesawat terbang yang fuselage-nya berdiameter 5 sampai 6 meter dan mempunyai lorong ganda. Penumpang biasanya duduk 7 sampai 10 sederet. Sebagai perbandingan, sebuah pesawat terbang berbadan sempit memiliki diameter 3 sampai 4 meter, dan memiliki lorong tunggal, dan diduduki 4 sampai 6 orang sederet. Pesawat...

ウズベキスタン共産党Коммунистическая партия УзбекистанаЎзбекистон Коммунистик партияси 創立 1925年解散 1991年11月3日後継政党 ウズベキスタン人民民主党政治的思想 共産主義マルクス・レーニン主義政治的立場 極左公式カラー 赤党旗 ウズベキスタンの政治ウズベキスタンの政党一覧ウズベキスタンの選挙 ウズベキスタン共産党 (ウズ...

lnمعلومات عامةنوع واجهة سطر الأوامرنظام التشغيل يونكس و شبيه يونكسالمطورون مختبرات بيلمعلومات تقنيةالإصدار الأول 3 نوفمبر 1971؛ منذ 52 سنة (1971-11-03)تعديل - تعديل مصدري - تعديل ويكي بيانات ال أن (يونكس) او ln command هو أداة مساعدة قياسية من يونكس تُستخدم لإنشاء رابط صلب أو و�...

لمعانٍ أخرى، طالع سميثفيلد (توضيح). سميثفيلد الإحداثيات 41°55′19″N 71°32′58″W / 41.921944444444°N 71.549444444444°W / 41.921944444444; -71.549444444444 تقسيم إداري البلد الولايات المتحدة[1][2] التقسيم الأعلى مقاطعة بروفيدانس خصائص جغرافية المساحة 72001669 متر م�...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Desember 2022. Xenocrasis fereyi Klasifikasi ilmiah Kerajaan: Animalia Filum: Arthropoda Kelas: Insecta Ordo: Coleoptera Famili: Cerambycidae Genus: Xenocrasis Spesies: Xenocrasis fereyi Xenocrasis fereyi adalah spesies kumbang tanduk panjang yang tergolong famili C...

О языке Гуандуна, Гонконга и Макао см. Юэ (язык). Кантонский диалект Самоназвание 广府话/廣府話 gwong2 fu2 waa2 广州话/廣州話 gwong2 zau1 waa2 白话/白話 baak6 waa2 В Гонконге и Макао:廣東話/广东话 gwong2 dung1 waa2 Страны КНР: центр и запад Гуандуна, восток Гуанси Гонконг Макао Австралия Канада: Ванкувер, Т�...

Este artigo contém a lista dos jogos da Seleção Brasileira de Futebol masculina de 2000 a 2019. Legenda Cor de fundo verde = Vitória da Seleção Brasileira Cor de fundo amarela = Empate Cor de fundo vermelha = Derrota da Seleção Brasileira Década de 2000 2000 Tailândia v Brasil 23 de fevereiro AmistosoJogo 697 Tailândia 0 – 7 Brasil Bangkok, Tailândia Rivaldo 13', 38'Ronaldinho Gaúcho 44'Émerson 50', 85'Roque Júnior 73'Jardel 80' Estádio: Estádio Raja...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Flags of micronations – news · newspapers · books · scholar · JSTOR (April 2023) (Learn how and when to remove this template message) Flags of micronations and intermicronational organisations at MicroCon 2022 Micronations are ephemeral, self-proclaimed entitie...

English poet and physician For other people named Richard Blackmore, see Richard Blackmore (disambiguation). Richard BlackmoreBorn22 January 1654Corsham, WiltshireDied9 October 1729 (aged 75)Boxted, EssexNationalityEnglishOccupation(s)poet, physician Sir Richard Blackmore (22 January 1654 – 9 October 1729), English poet and physician, is remembered primarily as the object of satire and as an epic poet, but he was also a respected medical doctor and theologian. Earlier years He was born at C...

Malaysian politician and field engineer In this Chinese name, the family name is Yeo (杨). Yang Berhormat PuanYeo Bee YinMP杨美盈Yeo Bee Yin in 2018Minister of Energy, Science, Technology, Environment and Climate ChangeIn office2 July 2018 – 24 February 2020MonarchsMuhammad V (2018–2019) Abdullah (2019–2020)Prime MinisterMahathir MohamadDeputyIsnaraissah Munirah MajilisPreceded byWilfred Madius Tangau (Science, Technology) Maximus Ongkili (Energy) Wan Junaidi Tuanku Jaafar ...

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (أكتوبر 2019) مخطط توضيحي للممارسات التصالحية الممارسات التصالحية هي علم اجتماعي يدرس كيفية تحسين وإصلاح العلاقات بين الناس والمجتمعات. والغرض من ذلك هو بناء مجتمعات صح...