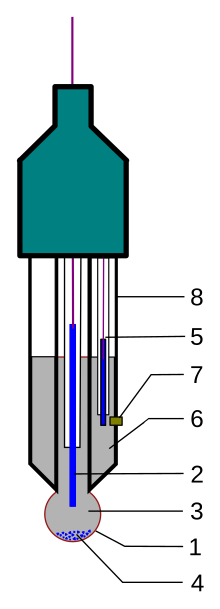
Glass electrode
|
Read other articles:

Australian judge The Right HonourableSir Frank KittoAC, KBE, QCHigh Court in 1952, Kitto far right, back rowJustice of the High Court of AustraliaIn office10 May 1950 – 1 August 1970Nominated byRobert MenziesPreceded bySir George RichSucceeded bySir Harry Gibbs Personal detailsBorn30 July 1903Melbourne, Victoria, AustraliaDied15 February 1994Armidale, New South Wales, Australia Sir Frank Walters Kitto, AC, KBE, QC (30 July 1903 – 15 February 19...

Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. L'introduction de cet article est soit absente, soit non conforme aux conventions de Wikipédia (novembre 2019). Ces motifs sont peut-être précisés sur la page de discussion. — Découvrez comment faire pour en améliorer la rédaction. Frontières temporaires créées par l'avancée des troupes allemandes et soviétiques. La frontière fut d’abord ajustée selon les termes du Pacte germano-soviétique conc...

Bandar Udara WunopitoWunopito AirportIATA: LWEICAO: WATWInformasiJenisPublikPemilikPemerintah IndonesiaPengelolaKementerian Perhubungan Republik IndonesiaMelayaniLewoleba, Nusa Tenggara Timur, IndonesiaKetinggian dpl5 mdplKoordinat08°21′45″S 123°26′17″E / 8.36250°S 123.43806°E / -8.36250; 123.43806Landasan pacu Arah Panjang Permukaan m kaki 02/20 1.200 3.937 Aspal Sumber: DAFIF[1][2] Bandar Udara Wunopito (bahasa Inggris: Wunop...

此生者传记没有列出任何参考或来源。 (2019年12月17日)请协助補充可靠来源,针对在世人物的无法查证的内容将被立即移除。 青田典子女演员原文名あおた のりこ罗马拼音Aota Noriko昵称泡沫青田别名森田典子、森陽子国籍 日本民族大和出生 (1967-10-07) 1967年10月7日(56歲) 日本愛媛縣松山市职业演員、藝人语言日語母校戶板女子短期大學肄業配偶玉置浩二出道日期1986年

American horror television series For similar terms, see Midnight Club and Midnight Club (film). The Midnight ClubGenre Horror Mystery-thriller Created by Mike Flanagan Leah Fong Based onThe Midnight Cluband other works of Christopher PikeStarring Iman Benson Igby Rigney Ruth Codd Annarah Cymone Chris Sumpter Adia Aya Furukawa Sauriyan Sapkota Matt Biedel Samantha Sloyan Zach Gilford Heather Langenkamp ComposerThe Newton BrothersCountry of originUnited StatesOriginal languageEnglishNo. of sea...

この記事の主題はウィキペディアにおける人物の特筆性の基準を満たしていないおそれがあります。基準に適合することを証明するために、記事の主題についての信頼できる二次資料を求めています。なお、適合することが証明できない場合には、記事は統合されるか、リダイレクトに置き換えられるか、さもなくば削除される可能性があります。出典検索?: 嶋野花...

Yū KamiyaInformación personalNacimiento 10 de noviembre de 1984 (39 años)Uberaba (Brasil)Nacionalidad Brasileña y japonesaInformación profesionalOcupación Escritor, ilustrador, mangaka y novelista ligero Obras notables Itsuka Tenma no Kuro UsagiNo Game No Life Sitio web ykp3.seesaa.net Firma [editar datos en Wikidata] Thiago Furukawa Lucas (nacido el 10 de noviembre de 1984),[1] que se hace llamar Yū Kamiya (榎宮祐, Kamiya Yū?), es un novelista, ilustrador[2]...

Nota: Para outros significados de La Unión, veja La Unión. Esta página cita fontes, mas que não cobrem todo o conteúdo. Ajude a inserir referências. Conteúdo não verificável pode ser removido.—Encontre fontes: ABW • CAPES • Google (N • L • A) (Dezembro de 2019) União Região Arequipa Capital Cotahuasi Prefeito População 17.200 habitantes Censo 2005 Área 4.746,40 km² Densidade 3,6 hab./km² Mapas Localizaçã...

Manga written by Garon Tsuchiya and Nobuaki Minegishi Old BoyCover to Vol. 1オールドボーイ―ルーズ戦記(Ōrudo Bōi Rūzu Senki)GenreAction[1]Psychological thriller[2] MangaWritten byGaron TsuchiyaIllustrated byNobuaki MinegishiPublished byFutabashaEnglish publisherNA: Dark Horse ComicsImprintAction ComicsMagazineManga ActionDemographicSeinenOriginal run1996 – 1998Volumes8 (List of volumes) Live-action films Oldboy (2003) Zinda (2006) Oldboy (2013)...

Railway station in Surrey, England FarnhamGeneral informationLocationFarnham, WaverleyEnglandGrid referenceSU844465Managed bySouth Western RailwayPlatforms2Other informationStation codeFNHClassificationDfT category C2HistoryOpened8 October 1849Passengers2017/18 1.563 million Interchange 8532018/19 1.563 million Interchange 1,0062019/20 1.548 million Interchange 25,3402020/21 0.274 million Interchange 6,2522021/22 0.931 million Interchange ...

Protestant Separatists from the Church of England A Catalogue of the Severall Sects and Opinions in England and other Nations: With a briefe Rehearsall of their false and dangerous Tenents, a propaganda broadsheet denouncing English dissenters from 1647, including Jesuits, Welsh blasphemers, Arminians, Arians, Adamites, Libertines (Antinomians), Antescripturians, Soul sleepers, Anabaptists, Familists, Seekers, and Divorcers English Dissenters or English Separatists were Protestants who separa...

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (فبراير 2019) ستانلي تايلور معلومات شخصية تاريخ الميلاد 2 مارس 1875 تاريخ الوفاة 22 يوليو 1965 (90 سنة) الجنسية المملكة المتحدة المملكة المتحدة لبريطانيا العظمى وأيرلند�...

South Korean actor In this Korean name, the family name is Park. Park Myung-hoonPark in August 2022Born (1975-05-28) May 28, 1975 (age 48)South KoreaOccupationActorYears active1999-presentAgentAce FactoryKorean nameHangul박명훈Revised RomanizationBak Myeong-hunMcCune–ReischauerPak Myŏnghun Park Myung-hoon (Korean: 박명훈; born May 28, 1975), is a South Korean actor. He made his acting debut as a stage actor in the 1999 play Class.[1] He is best known internationally...

В Википедии есть статьи о других людях с фамилией Ле-Блан. Жюльен Ле Блан Дата рождения 30 марта 1851(1851-03-30)[1][2][…] Место рождения Париж, Франция Дата смерти 28 февраля 1936(1936-02-28)[3] (84 года) Место смерти Париж, Франция Страна Франция[4] Медиафайлы на Викис...

State park in Lauderdale and Lawrence counties, Alabama, United States Joe Wheeler State ParkLocation in AlabamaLocationLauderdale & Lawrence counties, Alabama, United StatesCoordinates34°47′28″N 87°22′45″W / 34.79111°N 87.37917°W / 34.79111; -87.37917Area2,550 acres (10.3 km2)Elevation571 ft (174 m)DesignationAlabama state parkEstablished1949Named forJoseph Joe WheelerAdministratorAlabama Department of Conservation and Natural ResourcesW...

Hawker 400 adalah pesawat jet perusahaan kecil mesin kembar. Awalnya dirancang dan dibangun oleh Mitsubishi, telah dikembangkan lebih lanjut dan diperbarui oleh Beech Aircraft Company, sekarang bagian dari Hawker Beechcraft . Referensi Field, Hugh and Hurst, Mike. The Great St Louis Meeting. Flight International, 30 September 1978, pp. 1261–1266. Taylor, John W.R. (editor). Jane's All The World's Aircraft 1988-89. Coulsdon, UK: Jane's Defence Data, 1988. ISBN 0-7106-0867-5. Taylor, Michael ...

تحتاج هذه المقالة إلى الاستشهاد بمصادر إضافية لتحسين وثوقيتها. فضلاً ساهم في تطوير هذه المقالة بإضافة استشهادات من مصادر موثوقة. من الممكن التشكيك بالمعلومات غير المنسوبة إلى مصدر وإزالتها. عبد الله بن شداد معلومات شخصية تاريخ الوفاة 82 هـ الأب شداد بن الهاد الليثي الحي...

Species of harvestman/daddy longlegs Zuma tioga Briggs, 1971 (SDSU OP1048) Zuma tioga Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Arthropoda Subphylum: Chelicerata Class: Arachnida Order: Opiliones Family: Paranonychidae Genus: Zuma Species: Z. tioga Binomial name Zuma tiogaBriggs, 1971 Zuma tioga is a species of armoured harvestman in the family Paranonychidae.[1][2][3][4] It is found in North America.[1][5] Refer...

Pour le jeu dérivé, voir Buffy contre les vampires (jeu de cartes). Buffy contre les vampires Logo original de la série. Données clés Titre original Buffy the Vampire Slayer Genre Fantasy urbaineHorreurActionComédie dramatique Création Joss Whedon Production Producteurs exécutifs :Joss WhedonDavid GreenwaltMarti NoxonFran Rubel KuzuiKaz KuzuiSociétés de production :Mutant EnemySandollar TelevisionKuzui Entertainment20th Century Fox Television Acteurs principaux Sarah Miche...

هذه المقالة عن قرية ومنطقة إدارية في أفغانستان. لأسماء مشابهة، طالع جيلان (توضيح). جيلان (أفغانستان) تقسيم إداري البلد أفغانستان [1] إحداثيات 32°43′34″N 67°38′05″E / 32.7261°N 67.6347°E / 32.7261; 67.6347 الرمز الجغرافي 1140919 تعديل مصدري - تعديل جيلان هو اسم مد�...

![{\displaystyle E=E^{0}+{\frac {RT}{z_{i}F}}\ln \left[a_{i}+\sum _{j}\left(k_{ij}a_{j}^{z_{i}/z_{j}}\right)\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/173a9dcfbed1bd62faa8126ce218e846c1aabc03)