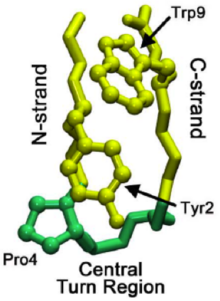
Beta hairpin
|
Read other articles:

Commuter rail station in Provo, Utah, US This article is about the FrontRunner commuter rail station. For the nearby Amtrak station, see Provo station (Amtrak). Provo Central 750 Provo Central station platformGeneral informationLocation690 South University Avenue[1]Provo, UtahUnited StatesCoordinates40°13′32″N 111°39′39″W / 40.22556°N 111.66083°W / 40.22556; -111.66083Owned byUtah Transit Authority (UTA)Platforms1 island platformTracks2Con...

يو-737 النوع غواصة من الفئة السابعة الجنسية ألمانيا النازية الشركة الصانعة شيتشاو-فيرك[1][2][3][4] المالك كريغسمارينه المشغل كريغسمارينه (30 يناير 1943–19 ديسمبر 1944)[1][3][4][5] المشغلون الحاليون وسيط property غير متوفر. المشغلون السابقون وسي�...

Vawoitung US-Bundesstoot: Oklahoma Sitz vo da Vawoitung: Cordell Adress vomVawoitungssitz: County CourthouseP.O. Box 380 Cordell, OK 73632-0380 Grindung: 1900 Buidt aus: Cheyenne-Land Vuawoi: 001 405 Demographie Eihwohna: 11.629 (2010) Dichtn: 4,5 Eihwohna/km² Eadkund Flächn gesamt: 2.613 km² Wossaflächn: 15 km² Koartn Koartn vo Washita County innahoib vo Oklahoma Washita County[1] is a County in Oklahoma in da USA. Beleg ↑ Washita County im Geographic Name...
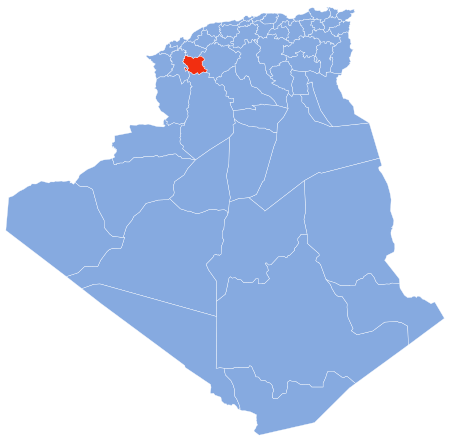
Commune and town in Saïda Province, AlgeriaOuled KhaledCommune and townCountry AlgeriaProvinceSaïda ProvinceTime zoneUTC+1 (CET) Ouled Khaled is a town and commune in Saïda Province in north-western Algeria.[1] References Algeria portal ^ Communes of Algeria. Statoids. Retrieved December 12, 2010. vte Saïda ProvinceCapital: SaïdaDistricts Aïn El Hadjar El Hassasna Ouled Brahim Saïda Sidi Boubekeur Youb Communes Aïn El Hadjar Aïn Sekhouna Aïn Soltane Doui Thabet El Hassa...

Potret resmi, 2019 John William Jay Raymond[1] adalah seorang jenderal Angkatan Antariksa Amerika Serikat yang menjabat sebagai kepala operasi antariksa pertamanya. Ia sebelumnya menjabat sebagai panglima Komando Antariksa Amerika Serikat, sebuah jabatan yang ia emban dari 29 Agustus 2019 sampai 20 Agustus 2020. Referensi ^ Clemson Commencement Program. Clemson.edu. May 1984. Diakses tanggal 2019-03-26. Artikel ini berisi bahan berstatus domain umum dari Pemerintah Am...

Аль-Ахлі Дата створення / заснування 1882[1] Менеджер/директор Suhaila Tarazid[2] Країна Палестинська держава Адміністративна одиниця Газа Власник Anglican Diocese of Jerusalemd, Південна баптистська конвенція і Church Mission Societyd Кількість лікарняних ліжок 80[3] Офіційний сай...

Menteroda Gemeinde Unstruttal Koordinaten: 51° 18′ N, 10° 34′ O51.30555555555610.562777777778431Koordinaten: 51° 18′ 20″ N, 10° 33′ 46″ O Höhe: 431 m Eingemeindung: 1. Januar 2023 Postleitzahl: 99996 Vorwahl: 036029 Menteroda (Thüringen) Lage von Menteroda in Thüringen Die Kirche von Menteroda Menteroda ist ein Ortsteil der Gemeinde Unstruttal im Unstrut-Hainich-Kreis in Thüringen. Inhaltsverzeichnis 1 Geographie ...

لوريالالشعارمعلومات عامةالجنسية فرنسا[1][2] التأسيس 1909 النوع عمل تجاري — مقاولة — شركة عمومية محدودة الشكل القانوني شركة عامة محدودة مع مجلس إدارة (n.o.s.)[3] المقر الرئيسي باريس فرنسا موقع الويب loreal.com المنظومة الاقتصاديةالشركات التابعة القائمة ... Kiehl's (en) لان

Kuil Bà Chúa Kho Bắc Ninh (listenⓘ) adalah sebuah kota di bagian utara Vietnam dan merupakan ibu kota Provinsi Bac Ninh. Kota ini adalah pusat budaya, administrasi dan komersial provinsi. Luas kota adalah 82,60 km persegi, dengan populasi 501.199 pada November 2017. Pada Januari 2006, kota (thị xã) Bắc Ninh ditingkatkan menjadi kota (thành phố). Pada Maret 1884, Bắc Ninh adalah situs kampanye yang menentukan dalam pertempuran antara Prancis dan berbagai Pasukan Bendera Hi...

{{{الاسم}}} بيانات المراقبة شاهد ايضا: تجمع مجري، العنقود المجري، قائمة مجموعات وعناقيد المجرات تعديل مصدري - تعديل 14 مجرة عملاقة من الصفيحة المحلية (منظر أمامي). الصفيحة المحلية في علم الفلك هي منطقة قريبة من الفضاء خارج المجري حيث تشترك درب التبانة وأعضاء المجموعة المحلي

Das Bayerische Konkordat (abgekürzt: „BayK“) vom 29. März 1924 ist ein Staatskirchenvertrag, der zwischen dem Freistaat Bayern und dem Heiligen Stuhl abgeschlossen wurde. Inhaltsverzeichnis 1 Vorgeschichte 2 Konkordatsverhandlungen 3 Der Inhalt des Konkordats von 1924 3.1 Kollektive Glaubensfreiheit 3.2 Hochschulen 3.3 Schulen 3.4 Fortgeltung des Konkordats von 1817 3.5 Ernennung von Geistlichen 4 Innerstaatliche Umsetzung 1925 5 Weitergeltung 5.1 Gemeinschaftsschule 5.2 Hochschulen 6 G...

Football clubMoneni Pirates FCFull nameMoneni Pirates Football ClubNickname(s)Buccaneers, BhakajujuFounded16 September 1967GroundMavuso StadiumManzini, EswatiniCapacity8 000[citation needed]LeaguePremier League of Eswatini2022–20237th Home colours Away colours Moneni Pirates FC is a Eswatinii soccer club based in Manzini. They play in the top division in Swazi football.[1] The team plays in white and black colors. History The club was established by Ngungunyane Matsenjwa, wi...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Nicktoons Racing – news · newspapers · books · scholar · JSTOR (February 2013) (Learn how and when to remove this template message) Not to be confused with Nicktoons Winners Cup Racing. 2000 video gameNicktoons RacingPAL PlayStation cover artDeveloper(s)Pipe Dr...

あべ じゅんこ阿部 純子 本名 阿部 純子(あべ じゅんこ)(旧姓)結婚後の姓は非公表別名義 吉永 淳(よしなが じゅん)生年月日 (1993-05-07) 1993年5月7日(30歳)出生地 日本 大阪府[1]出身地 大阪府身長 161 cm血液型 A型職業 女優・ファッションモデルジャンル 映画・テレビドラマ配偶者 一般男性(2022年 - )[2]事務所 アミューズ公式サイト 阿部 純子 - アミ�...

1982 filmThe Scorpion with Two TailsItalian theatrical release poster by Enzo Sciotti[2]Directed bySergio MartinoScreenplay by Ernesto Gastaldi Maria Chianetta[3] Story by Ernesto Gastaldi Dardano Sacchetti[3] Produced byLuciano Martino[1]Starring Elvire Audray Paolo Malco Claudio Cassinelli Marilù Tolo Wandisa Guida CinematographyGiancarlo Ferrando[3]Edited by Eugenio Alabiso Daniele Alabiso[3] Music byFabio Frizzi[3]Productioncompanie...

Indian screenwriter and film director Rajkumar R. PandeyPandey at Dulaara shootingBorn (1972-10-06) 6 October 1972 (age 51)Uttar Pradesh, IndiaNationalityIndianOther namesManoj R PandeyOccupationsFilm DirectorProducerScreenwriterMusic ComposerYears active1994–presentChildrenPradeep Pandey Rajkumar R. Pandey (born 6 October 1972) is an Indian film director, producer, music composer and screenwriter, known for his works in Bhojpuri films. He also big brother of action samrat Ma...

Restaurant chain Long John Silver's LLCCurrent logoTrade nameLong John Silver'sCompany typeSubsidiaryIndustryRestaurantsGenreFast-food restaurantFoundedAugust 18, 1969; 54 years ago (August 18, 1969)Lexington, Kentucky, United StatesFounderJim PattersonHeadquartersLouisville, Kentucky, U.S.Number of locations568 (as of June 17, 2023)Area servedUnited States Singapore Indonesia New Zealand Malaysia Philippines (Planned) Thailand (Planned) Vietnam (Planned) Kuwait (Planned) Japan (...

Not to be confused with Houghton-le-Spring. Chapel House Houghton-le-Side is a small village in the borough of Darlington and the ceremonial county of County Durham, England.[1] It is situated a few miles to the south-west of Newton Aycliffe.[1] The population at the 2011 Census was less than 100. Details are now maintained within the parish of Walworth. References ^ a b Ordnance Survey: Landranger map sheet 93 Middlesbrough (Darlington & Hartlepool) (Map). Ordnance Survey...
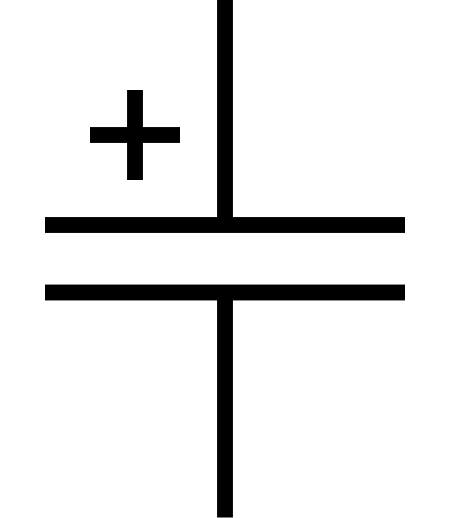
Эта статья или раздел нуждается в переработке.Пожалуйста, улучшите статью в соответствии с правилами написания статей. Обозначение на электрических схемах Электролити́ческие конденсаторы (оксидные) — разновидность конденсаторов, в которых диэлектриком между об�...

The HonorableHarry RoquePresidential SpokespersonIncumbentAssumed office April 13, 2020PresidentRodrigo DuterteDeputyChina JocsonPreceded bySalvador PaneloIn officeOctober 30, 2017 – October 15, 2018PresidentRodrigo DuterteDeputyChina JocsonPreceded byErnesto AbellaSucceeded bySalvador PaneloMember of the Philippine House of Representatives for Kabayan party-listIn officeJuly 25, 2016 – October 30, 2017Preceded byTerry RidonSucceeded byCiriaco Calalang Personal detai...