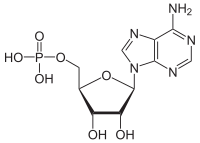
Adenosine monophosphate
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Prilly Mahatei Latuconsina, S.I.Kom. atau dikenal sebagai Prilly Latuconsina (lahir 15 Oktober 1996) adalah pemeran, presenter, penyanyi, model, pengusaha, aktivis, penulis, kreator konten dan produser eksekutif asal Indonesia berketurunan Ambon dan Sunda. Prilly memulai kariernya di dunia hiburan Indonesia pada tahun 2009. Prilly pertama kali muncul di layar kaca membawakan program Si Bolang untuk dua episode spesial Lombok, Nusa Tenggara Barat. Terjun di dunia entertainment sejak kecil dan ...

Fenibut Nombre IUPAC (RS)-4-amino-3-phenyl-butyric acidGeneralOtros nombres Fenibut, Phenybut, PhGABAFórmula estructural Fórmula molecular C10H13NO2 IdentificadoresNúmero CAS 1078-21-3[1]ChEBI 136039ChEMBL 315818ChemSpider 13491DrugBank 13455PubChem 14113UNII T2M58D6LA8 SMILESO=C(O)CC(c1ccccc1)CN InChIInChI=1S/C10H13NO2/c11-7-9(6-10(12)13)8-4-2-1-3-5-8/h1-5,9H,6-7,11H2,(H,12,13)Key: DAFOCGYVTAOKAJ-UHFFFAOYSA-N Propiedades físicasMasa molar 179,216 g/molPunto de ebullici�...

Martti Ahtisaari Martti Ahtisaari en 2012. Fonctions Président de la république de Finlande 1er mars 1994 – 1er mars 2000(6 ans) Élection 6 février 1994 Premier ministre Esko AhoPaavo Lipponen Prédécesseur Mauno Koivisto Successeur Tarja Halonen Ambassadeur de Finlande en Tanzanie 1973 – 1977(4 ans) Prédécesseur Seppo Pietinen Successeur Richard Müller Biographie Nom de naissance Martti Oiva Kalevi Ahtisaari Date de naissance 23 juin 1937 Lieu de naissance Viipuri (Finl...

Great Wall Great Wall Pao (seit 2019)Great Wall Pao (seit 2019) Pao Produktionszeitraum: seit 2019 Klasse: Utilities Karosserieversionen: Pick-up Motoren: Ottomotor:2,0–3,0 Liter(140–265 kW)Dieselmotoren:2,0–2,4 Liter(110–137 kW) Länge: 4963–5665 mm Breite: 1883–1991 mm Höhe: 1882–1970 mm Radstand: 2745–3470 mm Leergewicht: 2120 kg Heckansicht Der Great Wall Pao ist ein Pick-up-Modell des chinesischen Automobilherstellers Great Wall Motor, das seit 2...

FC Viktoria Köln Verein Vorlage:Infobox Fußballunternehmen/Wartung/Kein Bild Name FC Viktoria Köln 1904 e. V. Sitz Köln, Nordrhein-Westfalen Gründung 22. Juni 2010 Farben Schwarz-Weiß-Rot Mitglieder 623 (1. Juli 2019)[1] Vorstand Holger KirschWilly ScheerFranz Wunderlich Fußballunternehmen Vorlage:Infobox Fußballunternehmen/Wartung/Kein Bild Name FC Viktoria Köln 1904Spielbetriebs GmbH Gesellschafter 90,05 %: FC Viktoria Köln 1904 e. V.09,95 %: Franz-Jose...

2009 biographical sports drama film InvictusTheatrical release posterDirected byClint EastwoodScreenplay byAnthony PeckhamBased onPlaying the Enemy: Nelson Mandela and the Game that Made a Nation2008 bookby John CarlinProduced by Clint Eastwood Lori McCreary Robert Lorenz Mace Neufeld Starring Morgan Freeman Matt Damon CinematographyTom SternEdited by Joel Cox Gary D. Roach Music by Kyle Eastwood Michael Stevens ProductioncompaniesSpyglass Entertainment[1]Malpaso Productions[1]...

Mareau-aux-Prés La mairie. Administration Pays France Région Centre-Val de Loire Département Loiret Arrondissement Orléans Intercommunalité Communauté de communes des Terres du Val de Loire Maire Mandat Bertrand Hauchecorne 2020-2026 Code postal 45370 Code commune 45196 Démographie Gentilé Mareprésiens[1] Populationmunicipale 1 773 hab. (2020 ) Densité 133 hab./km2 Géographie Coordonnées 47° 50′ 52″ nord, 1° 48′ 01″ est Altitu...

Jahrbuchfoto der Northwestern Military and Naval Academy von Spencer Tracy (1919) Spencer Bonaventure Tracy (* 5. April 1900 in Milwaukee, Wisconsin; † 10. Juni 1967 in Beverly Hills, Kalifornien) war ein US-amerikanischer Filmschauspieler. Tracy, der seine Laufbahn auf der Bühne begann und später 20 Jahre lang zu den Spitzenstars von Metro-Goldwyn-Mayer gehörte, gilt als einer der größten Charakterdarsteller des 20. Jahrhunderts. Er gewann 1938 und 1939 den Oscar als bester ...

ستيف برتينشو (بالإنجليزية: Steve Burtenshaw) معلومات شخصية الميلاد 23 نوفمبر 1935(1935-11-23)[1]بورتسليد [لغات أخرى] الوفاة 17 فبراير 2022 (عن عمر ناهز 86 عاماً)وورثينغ [لغات أخرى] الطول 5 قدم 11 بوصة (1.80 م) مركز اللعب وسط الجنسية المملكة المتحدة مسي�...

Advanced Strategic Air-Launched Missile (ASALM) adalah sebuah program rudal strategis jarak menengah, yang dikembangkan pada akhir 1970-an untuk Angkatan Udara Amerika Serikat. Dimaksudkan untuk digunakan baik dalam peran udara-ke-permukaan dan anti-AWACS, pengembangan rudal mencapai tahap uji sistem propulsi sebelum dibatalkan pada tahun 1980. Referensi Parsch, Andreas (2003). Martin Marietta ASALM. Directory of U.S. Military Rockets and Missiles. designation-systems.net. Diarsipkan dari ver...

Famous large elephantThis article is about the historic elephant. For other uses, see Jumbo (disambiguation). Jumbo the Elephant redirects here. For the concrete and reinforced steel statue by Canadian artist Winston Bronnum, see Jumbo the Elephant (Bronnum). JumboJumbo and his keeper Matthew Scott(Circus poster, c. 1882)SpeciesAfrican bush elephantSexMaleBorn(1860-12-25)December 25, 1860[1]SudanDiedSeptember 15, 1885(1885-09-15) (aged 24)St. Thomas, Ontario, CanadaResting ...

G.D. Águias de Camarate Nome Grupo Desportivo Águias de Camarate Alcunhas ÁguiasCamarateÁguias de Camarate Torcedor(a)/Adepto(a) Camaratense Fundação 1 de agosto de 1950 (73 anos)[1]como Águias de Camarate Capacidade 1 500[2]como Águias de Camarate Localização Camarate, Portugal Presidente Frederico Dias Treinador(a) Marco Almeida Patrocinador(a) Orlecorte Material (d)esportivo Acerbis Competição Divisão de Honra[1] Taça de Portugal Taça da AF Lisboa Websit...

2010 British filmArchipelagoUK cinema release poster for ArchipelagoDirected byJoanna HoggWritten byJoanna HoggProduced byGayle GriffithsStarringTom HiddlestonKate FahyLydia LeonardAmy LloydChristopher BakerCinematographyEd RutherfordEdited byHelle Le FevreProductioncompanyWild Horses Film CompanyDistributed byArtificial Eye (UK)Release dates 22 October 2010 (2010-10-22) (London Film Festival) 4 March 2011 (2011-03-04) (UK & Ireland) Running time114 m...

This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this article by introducing citations to additional sources.Find sources: Limata – news · newspapers · books · scholar · JSTOR (November 2016) Limata was a Roman era city of Byzacena, in Roman North Africa.[1] It was home to the Bishop, Purpurius, one of the founders of Donatist Movement. References ^ Joseph Bingham...

Indian Cardinal of the Catholic Church His EminenceValerian GraciasPVCardinal, Archbishop of BombayCardinal Valerian Gracias greeted by members of the Indian Maltese community at the La Valette Band Club in Malta in 1958ArchdioceseArchdiocese of BombayProvinceBombayMetropolisBombaySeeBombayInstalled4 December 1950Term ended11 September 1978PredecessorArchbishop Thomas Roberts, S.J.SuccessorSimon PimentaOther post(s)Cardinal-Priest of Santa Maria in Via Lata(1953-1978) Auxiliary Bishop of Bomb...

Gilbert WarrentonWarrenton pada 1921Lahir(1894-03-07)7 Maret 1894Paterson, New Jersey, Amerika SerikatMeninggal21 Agustus 1980(1980-08-21) (umur 86)Riverside County, California, Amerika SerikatPekerjaanSinematografer Gilbert Warrenton (7 Maret 1894 – 21 Agustus 1980) adalah seorang sinematografer film bisu dan film bersuara Amerika Serikat. Ia memfilmkan lebih dari 150 film sebelum kematiannya. Ia adalah putra dari pemeran Lule Warrenton. Filmografi pilihan Kinkaid, Gambl...

Chemical compound O-1125Identifiers IUPAC name 6-[(6aR,10aR)-1-Hydroxy-6,6,9-trimethyl-6a,7,10,10a-tetrahydrobenzo[c]chromen-3-yl]-N,N,6-trimethylheptanamide PubChem CID9909689ChemSpider8085340Chemical and physical dataFormulaC26H39NO3Molar mass413.602 g·mol−13D model (JSmol)Interactive image SMILES CC1=CC[C@@H]2[C@@H](C1)C3=C(C=C(C=C3OC2(C)C)C(C)(C)CCCCC(=O)N(C)C)O InChI InChI=1S/C26H39NO3/c1-17-11-12-20-19(14-17)24-21(28)15-18(16-22(24)30-26(20,4)5)25(2,3)13-9-8-10-23(29)27(6)7/h11,...

Entryway area in Japanese buildings Genkan of a residence in Japan, viewed from outside looking in. The same genkan, viewed from inside looking out. The doors on the left wall are getabako. Genkan (玄関) are traditional Japanese entryway areas for a house, apartment, or building, a combination of a porch and a doormat.[1] It is usually located inside the building directly in front of the door. The primary function of genkan is for the removal of shoes before entering the main part o...

Glenn DennisBorn(1925-03-24)March 24, 1925Abilene, TexasDiedApril 28, 2015(2015-04-28) (aged 90)Roswell, New MexicoNationalityAmerican Glenn Dennis (March 24, 1925 – April 28, 2015) was a founder of the International UFO Museum and Research Center in Roswell, New Mexico. Early life In 1940, at age 15, Dennis began working as a part-time assistant at the Ballard Funeral Home, while attending Roswell High School. After graduating high school, Dennis was excused from wartime military serv...

Stock and station agencies are businesses which provide a support service to the agricultural community. Their staff who deal with clients are known as stock and station agents.[note 1] They advise and represent farmers and graziers in business transactions that involve livestock, wool, fertiliser, rural property and equipment and merchandise on behalf of their clients. The number and importance of these businesses fell in the late 20th century. Longridge Settlement – inspecting she...