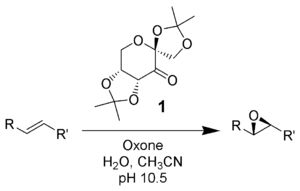
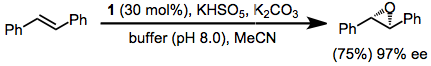
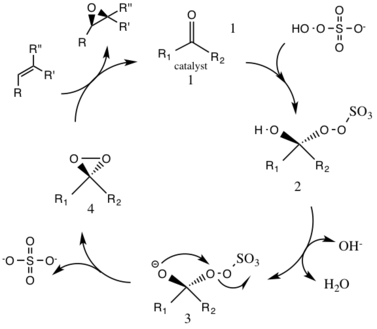
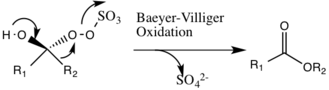
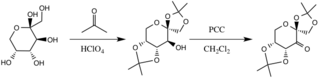
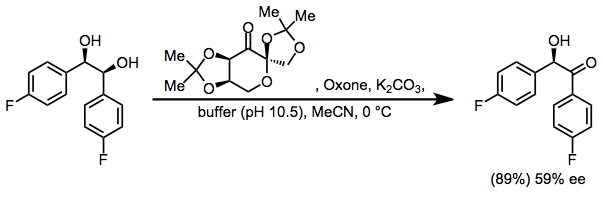
Shi epoxidation
| |||||||||||||||
Read other articles:

Sound systems that use 7 speakers and one subwooferLabel for 7.1 extended surround sound7.1 surround sound is the common name for an eight-channel surround audio system commonly used in home theatre configurations. It adds two additional speakers to the more conventional six-channel (5.1) audio configuration. As with 5.1 surround sound, 7.1 surround sound positional audio uses the standard front left and right, center, and LFE (subwoofer) speaker configuration. However, whereas a 5.1 surround...

Лунарные мифы — мифы о Луне и лунных циклах. Связаны с астральными мифами, прежде всего солярными: луна и солнце противопоставляются друг другу в рамках системы бинарных оппозиций, свойственной мифологическому сознанию (верх — низ, мужское — женское, живое — м

William Foden (en), 1918 Un guitariste est un musicien jouant de la guitare. Cet instrument est utilisé dans de nombreux styles comme la musique de la Renaissance, la musique baroque, la musique classique, le jazz, le rock ou le flamenco. Des guitaristes célèbres sont répertoriés dans les listes ci-dessous. Guitaristes classiques Article connexe : guitare classique. Guitaristes de la Renaissance Joueur de guitare assis dans un intérieur, P. Paolini, av. 1681 Les guitaristes de...

У Вікіпедії є статті про інших людей із прізвищем Качмарський. Яцек КачмарськийJacek Kaczmarski Яцек КачмарськийНародився 22 березня 1957(1957-03-22)[1][2][…]ВаршаваПомер 10 квітня 2004(2004-04-10)Гданськ·рак гортаніdПоховання Військові Повонзки : Громадянство ПольщаДіяльніс�...

Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. Cet article ne cite pas suffisamment ses sources (février 2020). Si vous disposez d'ouvrages ou d'articles de référence ou si vous connaissez des sites web de qualité traitant du thème abordé ici, merci de compléter l'article en donnant les références utiles à sa vérifiabilité et en les liant à la section « Notes et références » En pratique : Quelles sources sont attendues ? C...

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (نوفمبر 2019) ياكوب أولمان (بالألمانية: Jakob Ullmann) معلومات شخصية الميلاد 12 يوليو 1958 (65 سنة) فرايبرغ مواطنة ألمانيا الحياة العملية المهنة أستاذ جامعي، وملح�...

Cet article est une ébauche concernant les coléoptères. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Calosoma elegans Calosoma elegansClassification Règne Animalia Embranchement Arthropoda Classe Insecta Ordre Coleoptera Famille Carabidae Sous-famille Carabinae Tribu Carabini Genre Calosoma Sous-genre Calostoma (Callisthenes) EspèceCalosoma elegans(Kirsch, 1859) Géhin[1], 1885 Taxons de rang inférieur S...

2000 television film by Roger Young This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: The Thin Blue Lie – news · newspapers · books · scholar · JSTOR (October 2023) (Learn how and when to remove this template message) The Thin Blue LieWritten byDaniel HelfgottDirected byRoger YoungStarringRob MorrowRandy Quaid...

Dr. (H.C.) K. H.Idham ChalidWakil Perdana Menteri IndonesiaMasa jabatan24 Maret 1956 (1956-03-24) – 9 Juli 1959 (1959-07-9)PresidenSoekarnoPerdana MenteriAli SastroamidjojoDjoeanda KartawidjajaMenjabat bersama Daftar Mohamad Roem (1956–1957)Johannes Leimena (1957–1959)Hardi (1957–1959) PendahuluDjanoe IsmadiHarsono TjokroaminotoPenggantiJohannes LeimenaMasa jabatan24 Februari 1966 (1966-02-24) – 27 Maret 1966 (1966-03-27)Perdana MenteriSoekarnoM...

US Navy admiral Admiral Dewey redirects here. For the boat, see Admiral Dewey (tugboat). This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: George Dewey – news · newspapers · books · scholar · JSTOR (October 2023) (Learn how and when to remove this template message) Admiral of the NavyGeorge DeweyDewey in 1899B...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: List of accidents and incidents involving military aircraft 1943–1944 – news · newspapers · books · scholar · JSTOR (July 2013) (Learn how and when to remove this template message) This is a list of accidents and incidents involving military aircraft gro...

Rusma Yul AnwarFoto sebagai bupati, 2021Bupati Pesisir Selatan ke-17PetahanaMulai menjabat 26 Februari 2021WakilRudi Hariyansyah (2021–23)[1]PendahuluHendrajoniMuskamal (pelaksana harian)Wakil Bupati Pesisir Selatan ke-4Masa jabatan17 Februari 2016 – 17 Februari 2021PendahuluEditiawarmanPenggantiRudi Hariyansyah Informasi pribadiLahir30 Juli 1963 (umur 60)Painan, Sumatera Barat, IndonesiaKebangsaanIndonesiaPartai politik Gerindra (sampai 2022)&...

Koordinat: 2°3′32″S 101°23′29″E / 2.05889°S 101.39139°E / -2.05889; 101.39139 Kota Sungai Penuh SungaipenuhKotaTranskripsi bahasa daerah • Abjad Jawiسوڠاي ڤنوهMasjid Agung Pondok Tinggi LambangJulukan: Kota SaktiKota SejukMotto: Sahalun suhak, salatuh bdei(Kerinci) Kompak dan berlandasan mufakatPetaKota Sungai PenuhPetaTampilkan peta SumatraKota Sungai PenuhKota Sungai Penuh (Indonesia)Tampilkan peta IndonesiaKoordinat: 2...

Highway in Mississippi Mississippi Highway 365MS 365 highlighted in pinkRoute informationMaintained by MDOTLength23.278 mi[1] (37.462 km)Existed1950–presentMajor junctionsSouth end MS 30 in BurtonMajor intersections US 72 near BurnsvilleNorth end MS 25 near Pickwick Lake LocationCountryUnited StatesStateMississippiCountiesPrentiss, Tishomingo Highway system Mississippi State Highway System Interstate US State ← MS 364→ MS 366 Mis...

Shunting Locomotive Yorkshire Engine Company Half JanusA Yorkshire Engine Company Half Janus preserved at the Appleby Frodingham Railway Preservation Society at Scunthorpe SteelworksType and originPower typeDiesel-electricBuilderYorkshire Engine CompanyModel0-6-0DEBuild date1956–1965SpecificationsConfiguration: • UICCGauge1,435 mm (4 ft 8+1⁄2 in)Loco weight31 long tons (31.5 t)Prime moverRolls-Royce C6SFLTraction motorsBritish Thomson-Houst...

Australian rules footballer Australian rules footballer Bradd Dalziell Dalziell in 2014Personal informationFull name Bradd DalziellNickname(s) Razzle DazzleDate of birth (1987-03-15) 15 March 1987 (age 36)Place of birth Western AustraliaOriginal team(s) Lynwood Ferndale JFCDraft 52nd, 2007 National Draft (Brisbane Lions)Height 184 cm (6 ft 0 in)Weight 82 kg (181 lb)Position(s) MidfielderClub informationCurrent club East Fremantle Football ClubPlaying ca...

For the village of the same name, see Widawa, Łódź Voivodeship. This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Widawa – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this template message) River in PolandWidawaThe Widawa near WrocławLocationCountryPolandPhysical characteri...

1973 live album by Major LanceMajor Lance's Greatest Hits Recorded Live at the TorchLive album by Major LanceReleased1973RecordedStoke-on Trent 1972GenreSoulLabelContempo COLP1001ProducerMajor LanceMajor Lance chronology Um, Um, Um, Um, Um, Um - The Best of Major Lance(1964) Major Lance's Greatest Hits Recorded Live at the Torch(1973) Now Arriving(1978) Professional ratingsReview scoresSourceRatingAllMusic[1]The Encyclopedia of Popular Music[2] Major Lance's Greatest H...

الإعذاب أو إزالة الملوحة[1] أو تحلية المياه أو الزّملحة[2] هي سلسلة من العمليات الصناعية تجرى لإزالة كل أو جزء من الأملاح الزائدة والمعادن من المياه. وقد يستخدم هذا المصطلح إلى إزالة الأملاح والمعادن الذائبة في الماء. ويمكن تحلية مياه البحر لتصبح من الممكن استخدامها...

Terminal BayuanggaTerminal Penumpang Tipe A Kode: BYA Papan Nama Terminal BayuanggaLokasiJalan Bromo Nomor 18, Kelurahan Triwung Lor, Kecamatan Kademangan, Kota Probolinggo, Provinsi Jawa Timur, Kodepos 67223 IndonesiaKoordinat7°45′58″S 113°10′28″E / 7.766168°S 113.174383°E / -7.766168; 113.174383Koordinat: 7°45′58″S 113°10′28″E / 7.766168°S 113.174383°E / -7.766168; 113.174383Pemilik Pemerintah Kota ProbolinggoOpera...