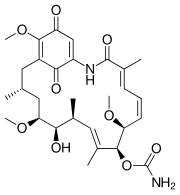
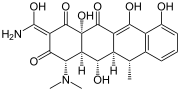
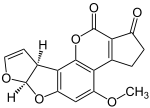
Polyketide
|
Read other articles:

В Википедии есть статьи о других людях с фамилией Росс. Необходимо проверить качество перевода, исправить содержательные и стилистические ошибки. Вы можете помочь улучшить эту статью (см. также рекомендации по переводу).Оригинал на английском языке — Art Ross. Арт Росс Пози�...

جغرافيا إسبانيامعلومات عامةالبلد إسبانيا القارة أوروبا (ينطبق على peninsular Spain (en) ، جزر البليار)إفريقيا (ينطبق على مليلية، سبتة، جزر الكناري، بلاثاس دي سوبيرانیا) الساحل المحيط الأطلسيالبحر الأبيض المتوسطبحر قنطبريةبحر البوران الحدود أندوراالبرتغالالمغربفرنساجبل طا

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (أغسطس 2022) لمعانٍ أخرى، طالع الجسر (توضيح). الجسر النوع دراما تلفزيونية [لغات أخرى]، ومسلسل جريمة [لغات أخرى][1]، ومسلسل إثارة &...

Enrico LettaPerdana Menteri ItaliaMasa jabatan28 April 2013 – 22 Februari 2014PresidenGiorgio NapolitanoWakilAngelino AlfanoPendahuluMario MontiPenggantiMatteo RenziMenteri PertanianMasa jabatan27 Januari 2014 – 22 Februari 2014PendahuluNunzia De GirolamoPenggantiMaurizio MartinaSekretaris Dewan MenteriMasa jabatan17 Mei 2006 – 8 Mei 2008Perdana MenteriRomano ProdiPendahuluGianni LettaPenggantiGianni LettaMenteri PerindustrianMasa jabatan22 Desember 1999 ...

English translation of the Bible New American Bible Revised EditionFull nameNew American Bible Revised EditionAbbreviationNABREComplete BiblepublishedMarch 9, 2011Derived fromConfraternity Bible, New American BibleTextual basisOT (2011 revision): Biblia Hebraica Stuttgartensia[citation needed] with Dead Sea Scrolls and minor Septuagint influence. Deuterocanonicals: Septuagint, Dead Sea Scrolls, and some Vulgate. NT: (1986 revision): UBS3, the third edition of United Bible Societies' T...

Butir-Butir Budaya Jawa PengarangJenderal Purnawirawan SoehartoNegaraIndonesiaBahasaJawa, Indonesia, dan InggrisGenreDidaktikPenerbit• Yayasan Purna Bhakti Pertiwi (Indonesia)Tanggal terbit1987 Butir-Butir Budaya Jawa, adalah sebuah buku yang ditulis oleh (mantan) Presiden RI Soeharto. Buku ini pertama kali terbit pada tahun 1987. Buku ini berisi ajaran-ajaran kehidupan. Meskipun pertama kali terbit pada tahun 1987, menurut kolofon, buku ini sudah selesai ditulis pada tahun 1983. Subju...

Dutch politician Faissal BoulakjarMember of the House of RepresentativesIn office31 March 2021 – 5 December 2023Member of the Breda municipal councilIn office27 March 2014[1] – 1 April 2021[2]Succeeded byBart Lauwen Personal detailsBornF. Boulakjar (1979-04-28) 28 April 1979 (age 44)Tifarouine, MoroccoPolitical partyDemocrats 66Children3Alma materSpectrum College Faissal Boulakjar (born 28 April 1979) is a Moroccan-born Dutch politician, who has been...

Russian author and medical doctor (1891–1940) In this name that follows Eastern Slavic naming conventions, the patronymic is Afanasyevich and the family name is Bulgakov. Mikhail BulgakovBulgakov in 1928BornMikhail Afanasyevich Bulgakov15 May [O.S. 3 May] 1891Kiev, Kiev Governorate, Russian EmpireDied10 March 1940(1940-03-10) (aged 48)Moscow, Russian SFSR, Soviet UnionResting placeNovodevichy Cemetery, MoscowOccupationNovelist, short-story writer, playwright, phys...

Lego theme Lego Minions: The Rise of GruSubjectMinions: The Rise of GruLicensed fromUniversal Pictures and IlluminationAvailabilityMarch 2020–December 2022Total sets7[1]CharactersBelle Bottom, Bob, Gru, Kevin, Otto and StuartOfficial website Lego Minions: The Rise of Gru (stylized as LEGO Minions: The Rise of Gru) was a Lego theme based on the film of the same name. It is licensed from Universal Pictures and Illumination.[2] The theme was first introduced in March ...

Blue ChristmasLagu oleh Elvis Presleydari album Elvis' Christmas AlbumSisi-BWooden Heart (447-0720)Santa Claus Is Back in Town (447-0647)Dirilis09 November 1964 (1964-11-09) (447-0720)26 November 1965 (1965-11-26) (447-0647)Format7-inchDirekam05 September 1957 (1957-09-05), Radio Recorders, Hollywood, CaliforniaGenre Natal rhythm and blues Durasi2:07LabelRCA Victor 447-0720RCA Victor 447-0647Pencipta Billy Hayes Jay W. Johnson Blue ChristmasLagu oleh The Beach Boysdari album Th...

Danish handball player (born 1992) Louise Burgaard Personal informationFull name Louise Katharina Vinter BurgaardBorn (1992-10-17) 17 October 1992 (age 31)Esbjerg, DenmarkNationality DanishHeight 1.76 m (5 ft 9 in)Playing position Right backClub informationCurrent club Metz HandballNumber 19Senior clubsYears Team2008–2011 KIF Vejen2011–2013 TTH Holstebro2013–2015 Viborg HK2015–2019 FCM Håndbold2019–2024 Metz Handball2024– Odense HåndboldNational teamYears Tea...

Iones y el potencial de membrana Se conoce como electrobiogénesis a la producción, en el curso de un proceso biológico, de una diferencia de potencial (tensión eléctrica) local independientemente de si es artificial o natural.[1] Se ha establecido que en cada célula viva existe de modo permanente, entre la parte interna y la parte externa de su membrana, una diferencia de potencial eléctrica del orden de una decena de milivoltios, que se incrementa en períodos de actividad (lla...

Soccer clubOlé BrasilFull nameOlé Brasil Futebol ClubeNickname(s)OléFounded21 September 2006; 17 years ago (2006-09-21)GroundEstádio Palma TravassosCapacity17,360PresidentEduardo Zanello2015Paulistão 2ª Divisão, 14th of 30 Home colors Away colors Olé Brasil Futebol Clube, usually known as Olé Brasil, is a currently inactive Brazilian football club based in Ribeirão Preto, a city in the state of São Paulo. History The club was founded on September 21, 2006 by entre...

This article relies excessively on references to primary sources. Please improve this article by adding secondary or tertiary sources. Find sources: British School Jakarta – news · newspapers · books · scholar · JSTOR (January 2010) (Learn how and when to remove this template message) School in IndonesiaBritish School JakartaLocationBintaro Sektor 9Jl. Raya Jombang-CiledugPerigi Lama, Pondok ArenSouth Tangerang 15427IndonesiaCoordinates6°16′23″S 1...

Bagian dari seri mengenai Sejarah Indonesia Prasejarah Manusia Jawa 1.000.000 BP Manusia Flores 94.000–12.000 BP Bencana alam Toba 75.000 BP Kebudayaan Buni 400 SM Kerajaan Hindu-Buddha Kerajaan Kutai 400–1635 Kerajaan Tarumanagara 450–900 Kerajaan Kalingga 594–782 Kerajaan Melayu 671–1347 Kerajaan Sriwijaya 671–1028 Kerajaan Sunda 662–1579 Kerajaan Galuh 669–1482 Kerajaan Mataram 716–1016 Kerajaan Bali 914–1908 Kerajaan Kahuripan 1019&#...

Catholic archdiocese in France Archdiocese of Aix-en-Provence and ArlesArchidioecesis Aquensis in Gallia et Arelatensis Archidiocèse d'Aix-en-Provence et Arles Archidiocèsi de z-Ais e Arle Archidioucèsi de z'Ais e Arle Aix CathedralLocationCountryFranceEcclesiastical provinceMarseilleMetropolitanArchdiocese of MarseilleStatisticsArea4,580 km2 (1,770 sq mi)Population- Total- Catholics(as of 2015)879,000 (est.)723,200 (est.)[citation needed] (82.3%)Paris...

Amusement ride This article is about a type of amusement ride. For the original example, first erected in Chicago in 1893, see Ferris Wheel (1893). For other uses, see Ferris wheel (disambiguation). Giant wheel redirects here. For other uses, see Giant Wheel (disambiguation). Ain Dubai, the tallest Ferris wheel in the world A Ferris wheel (also called a Giant Wheel or an observation wheel) is an amusement ride consisting of a rotating upright wheel with multiple passenger-carrying components ...

Опис файлу Опис Обкладинка альбому Мелані Чісхолм — «The Sea» Джерело Eng Wiki Час створення 2011 Автор зображення Невідомий Ліцензія див. нижче Обґрунтування добропорядного використання Обґрунтування добропорядного використання не вказано назву статті [?] Опис Обклад�...

Untuk keuskupan bernama sama dalam Gereja Swedia, lihat Keuskupan Stockholm (Gereja Swedia). Keuskupan StockholmDioecesis HolmiensisStockholms katolska stiftKatolik LokasiNegaraSwediaProvinsi gerejawiSubyek Langsung Tahta SuciKoordinat59°18′50″N 18°04′21″E / 59.31389°N 18.07250°E / 59.31389; 18.07250Koordinat: 59°18′50″N 18°04′21″E / 59.31389°N 18.07250°E / 59.31389; 18.07250StatistikLuas450.000 km2 (170.000 sq&...

Village in Lesser Poland Voivodeship, PolandŁaganówVillageŁaganówCoordinates: 50°11′N 20°17′E / 50.183°N 20.283°E / 50.183; 20.283Country PolandVoivodeshipLesser PolandCountyProszowiceGminaProszowice Łaganów [waˈɡanuf] is a village in the administrative district of Gmina Proszowice, within Proszowice County, Lesser Poland Voivodeship, in southern Poland. It lies approximately 3 kilometres (2 mi) south-west of Proszowice and 29 km (18 ...