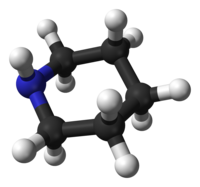
Piperidine
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Charity regulator in England and Wales Charity Commission redirects here. For other uses, see Charities Commission (disambiguation). Charity Commission forEngland and WalesWelsh: Comisiwn Elusennau Cymru a LloegrNon-ministerial government department overviewFormed1853 (1853)JurisdictionEngland and WalesHeadquartersPetty France, LondonEmployees420Annual budget£22.9 million (2016–17)[1]Non-ministerial government department executivesHelen Stephenson CBE, Chief Executive OfficerO...

Breakthru'Album studio karya NidjiDirilis1 September 2005 1 Juli 2007 (Versi Bahasa Inggris) 2 Januari 2016 (Versi Piringan Hitam)GenreBritPop, Alternative RockLabelMusica Studio'sProduserNoeyKronologi Nidji Breakthru' (2006) Breakthru' English Version (2007) Top Up (2007)Top Up2007 Sampul alternatif Breakthru' adalah album musik pertama karya Nidji. Dirilis tahun 2006 yang berisi 10 buah lagu dengan hits singel lagu Sudah, Hapus Aku, dan Kau dan Aku. Versi bahasa Inggrisnya dirilis pada ...

شاكر الفحام معلومات شخصية الميلاد سنة 1921 حمص تاريخ الوفاة 28 يونيو 2008 (86–87 سنة) الجنسية سوريا عضو في مجمع اللغة العربية بدمشق الزوجة مديحة عنبري أقرباء أحمد زويل (صهر) مناصب عضو مجلس الشعب السوري في المنصب1971 – 1973 نائب رئيس مجمع اللغة العربية بد

Ceylonese politician Pathiraja Mudiyanselage Kapuruhamy Tennekoon (born 16 May 1917) was a Ceylonese politician. He was the member of Parliament of Sri Lanka from Mihintale representing the Sri Lanka Freedom Party.[1][2] He was defeated in the 1977 general election. References ^ Hon. Tennekoon, Pathiraja Mudiyanselage Kapuruhamy, M.P. Directory of Past Members. Parliament of Sri Lanka. Retrieved 24 August 2021. ^ Parliament of Sri Lanka (1972). Members of the Legislatures of C...

Family of true bugs Thaumastocoridae Cimicomorph Bug (Thaumastocoridae spp.) Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Arthropoda Class: Insecta Order: Hemiptera Suborder: Heteroptera Infraorder: Cimicomorpha Superfamily: Miroidea Family: ThaumastocoridaeKirkaldy, 1908 Thaumastocoridae is a family of true bugs in the order Hemiptera. There are about 9 genera and more than 20 described species in the family Thaumastocoridae.[1][2][3] Genera T...

King of Imereti in western Georgia George IIGeorge II, a fresco from the Gelati MonasteryKing of Imereti (more...) Reign1565–1585PredecessorBagrat III of ImeretiSuccessorLevan of ImeretiDied1585SpouseRusudan Shervashidze Tamar DiasamidzeIssueLevan of ImeretiDynastyBagrationi dynastyFatherBagrat III of ImeretiMotherEleneReligionGeorgian Orthodox ChurchKhelrtva George II (Georgian: გიორგი II) (died 1585), of the Bagrationi dynasty, was a king of Imereti from 1565 to 1585. Reig...

العلاقات البوسنية البلغارية البوسنة والهرسك بلغاريا البوسنة والهرسك بلغاريا تعديل مصدري - تعديل العلاقات البوسنية البلغارية هي العلاقات الثنائية التي تجمع بين البوسنة والهرسك وبلغاريا.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومر

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (أغسطس 2023) ماريون ماهوني غريفينبيانات شخصيةالميلاد 14 فبراير 1871شيكاغوالوفاة 10 أغسطس 1961[1][2][3] (90 سنة) شيكاغو[4] مكان الدفن مقبرة غريسلاند بلدان المواطنة �...

American inorganic chemist (1928–2021) Fred HawthorneBornMarion Frederick Hawthorne(1928-08-24)August 24, 1928Fort Scott, KansasDiedJuly 8, 2021(2021-07-08) (aged 92)NationalityAmericanAlma materMissouri School of Mines and Metallurgy Pomona College (B.A., 1949)University of California, Los Angeles (Ph.D., 1953)Known forBoron hydridesAwardsTolman Award (1986)King Faisal International Prize (2003) Priestley Medal (2009) National Medal of Science (2011)Scientific careerFieldsIn...

2013 video game 2013 video gameStar TrekCover art featuring Spock (left) and KirkDeveloper(s)Digital ExtremesPublisher(s)Bandai Namco EntertainmentWriter(s)Marianne KrawczykComposer(s)Chad SeiterPlatform(s)PlayStation 3, Xbox 360, Microsoft WindowsReleaseNA: April 23, 2013EU: April 26, 2013Genre(s)Action-adventure, third-person shooterMode(s)Single-player, multiplayer Star Trek is a third-person action-adventure Star Trek video game. It was developed by Digital Extremes and co-published by Ba...

Asia's Next Top Model, Siklus 3Peserta Asia's Next Top Model (musim 3)Negara asal SingapuraJumlah episode13RilisSaluran asliSTAR WorldTanggal tayang25 Maret –17 Juni 2015Kronologi← SebelumnyaSiklus 2 Selanjutnya →Siklus 4 Asia's Next Top Model, Siklus 3 atau disingkat AsNTM3 adalah musim ketiga dari Asia's Next Top Model. Acara ini memperebutkan predikat sebagai Asia's Next Top Model. Acara ini menampilkan model-model potensial yang berasal dari berbagai negara-negar...

2021 American horror television miniseries Midnight MassPromotional posterGenre Drama Supernatural horror Created byMike FlanaganWritten by Mike Flanagan James Flanagan Elan Gale Dani Parker Jeff Howard Directed byMike FlanaganStarring Kate Siegel Zach Gilford Kristin Lehman Samantha Sloyan Igby Rigney Rahul Kohli Annarah Cymone Annabeth Gish Alex Essoe Rahul Abburi Matt Biedel Michael Trucco Crystal Balint Louis Oliver Henry Thomas Hamish Linklater Theme music composerThe Newton BrothersCoun...

Retailer Toolstation LimitedTypesubsidiaryIndustryRetailFounded2003FounderAlex MathersHeadquartersBridgwater, Somerset, United KingdomArea servedUnited KingdomNetherlandsFranceGermanyBelgiumKey peopleAngela Rushforth (Managing Director)Productstools, building materialsRevenue £633 million (2020) [1]OwnerTravis Perkins Group PlcNumber of employees5000 (2021)ParentTravis Perkins plcWebsitetoolstation.com Toolstation is a multi-channel retailer of tools and building materials. It has mo...

Defensive shield wall used by Roman Legions Roman soldiers in tortoise formation Part of a series on theMilitary of ancient Rome 753 BC – AD 476 Structural history Army Unit types and ranks Decorations and punishments Legions Auxilia Generals Navy Fleets Admirals Campaign history Wars and battles Technological history Military engineering Castra Siege engines Triumphal arches Roads Political history Strategy and tactics Infantry tactics Frontiers and fortifications Limes Limes Britann...

GianyarKecamatanPeta lokasi Kecamatan GianyarNegara IndonesiaProvinsiBaliKabupatenGianyarPemerintahan • CamatI Komang Alit Adnyana (Pj)[1]Luas • Total50,59 km2 (19,53 sq mi)Populasi (2021)[2] • Total99.979 jiwa • Kepadatan1.939/km2 (5,020/sq mi)Kode pos80511 – 80515Kode area telepon+62 361Kode Kemendagri51.04.03 Desa/kelurahan5 kelurahan12 desa Gianyar (Bali: aksara Bali: ᬕ᭄ᬬᬜᬃ, trans...

American politician Zaynab MohamedMember of the Minnesota Senatefrom the 63rd districtIncumbentAssumed office January 3, 2023Preceded byPatricia Torres Ray Personal detailsBorn (1997-05-04) May 4, 1997 (age 26)[1]SomaliaPolitical partyDemocratic (DFL)EducationUniversity of Minnesota (BA) Zaynab M. Mohamed (born May 4, 1997) is an American politician serving as a member of the Minnesota State Senate since 2023. A member of the Minnesota Democratic–Farmer–Labor Party (DFL),...

François I beralih ke sini. Untuk adipati Brittany, silakan lihat François I, Adipati Brittany Potret Francois I François I dari Prancis (12 September 1494 – 31 Juli 1547) ialah Raja Prancis dan anggota dan anggota Dinasti Valois. Dibawah pemerintahannya dibentuk Persekutuan Prancis-Usmaniyah dengan Suleiman I. Kelahiran François dilahirkan di Cognac, yang berada di Prancis pada 12 September 1494. Orang tuanya ialah Charles, Adipati Angouleme dan Louise dari Savoy. Pernika...

Perang WiskiKomandan Per Starklint dari HDMS Triton Denmark di Pulau Hans pada Agustus 2003Tanggal1973–14 Juni 2022LokasiPulau HansKoordinat80°49′35″N 66°27′30″W / 80.826389°N 66.458333°W / 80.826389; -66.458333Koordinat: 80°49′35″N 66°27′30″W / 80.826389°N 66.458333°W / 80.826389; -66.458333Nama lainKonflik batas wilayah Denmark-KanadaJenisSengketa wilayahPeserta/Pihak terlibat Denmark Kanada OlovHasilPulau H...

Armenian dynasty of Ottoman architects This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Balyan family – news · newspapers · books · scholar · JSTOR (August 2011) (Learn how and when to remove this template message) Graves of the Balyan family in the Armenian cemetery on Nuh Kuyusu Caddesi, Bağlarbaşı, Üsk...

Etruscan mythological figure For the moon, see Vanth (moon). Vanth in a fresco in an Etruscan tomb in Tarquinia Vanth is a chthonic figure in Etruscan mythology shown in a variety of forms of funerary art, such as in tomb paintings and on sarcophagi.[1] Background Vanth is a female demon in the Etruscan underworld that is often accompanied either by additional Vanth figures or by another underworld demon, Charun (later referred to as Charu). Both Vanth and Charun are only seen in icon...