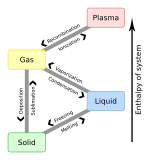
Latent heat
|
Read other articles:

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (يناير 2016) فردريش غوتليب ديتريش معلومات شخصية الميلاد 9 مارس 1765(1765-03-09)، 9 مارس 1768 الوفاة 2 يناير 1850 (84 سنة)أيسناخ مواطنة الإمبراطورية الرومانية المقدسة، الاتحاد الألماني...

VrbnicaВрбница Localização País Sérvia Província Sérvia central Distrito Zlatibor Município Sjenica Características geográficas População total (2011) 137 hab. Altitude 948 m Código postal Vrbnica (em cirílico: Врбница) é uma vila da Sérvia localizada no município de Sjenica, pertencente ao distrito de Zlatibor. A sua população era de 137 habitantes segundo o censo de 2011.[1][2] Demografia Evolução demográfica 19481953196119711981199120022011 182...

Olympische Jugend-Sommerspiele 2018Tennis Information Austragungsort Buenos AiresArgentinien Argentinien Wettkampfstätte Buenos Aires Lawn Tennis Club Nationen 32 Athleten 64 (32 , 32 ) Datum 7. bis 14. Oktober 2018 Entscheidungen 5 ← Nanjing 2014 Dakar 2022 → Bei den Olympischen Jugend-Sommerspielen 2018 in Buenos Aires wurden vom 7. bis zum 14. Oktober fünf Wettbewerbe im Tennis ausgetragen. Wettkampfstätte war der Buenos Aires Lawn Tennis Club. Inhaltsverzei...

Manuel Barrio Ayuso Información personalNacimiento 1788 o 12 de septiembre de 1786 Casarejos (España) Fallecimiento 23 de noviembre de 1850 Murcia (España) Nacionalidad EspañolaInformación profesionalOcupación Político Cargos ocupados Diputado de EspañaSenador de España Firma [editar datos en Wikidata] Manuel Barrio Ayuso (Casarejos, 1788 - Murcia, 23 de julio de 1850) fue un político español. Biografía Ministro de Gracia y Justicia en 1836 en el gabinete de Francisco Ja...

Премія Муз-ТВрос. Премия Муз-ТВ Країна РосіяТип статуетка у вигляді срібної тарілки з золотим яблуком, яке представлене у вигляді глобусаСтатус вручається Нагородження Параметри матеріал: срібло, золотоЗасновано: 5 червня 2003Останнє: 8 червня 2018Нагороджені: Черговість

American college football season 1891 VMI Keydets footballConferenceIndependentRecord3–0–1Head coachWalter Taylor (1st season)Seasons← 18731892 → 1891 Southern college football independents records vte Conf Overall Team W L T W L T Trinity (NC) – 3 – 0 – 0 Wake Forest – 1 – 0 – 0 VMI – 3 – 0 – 1 Vanderbilt – 3 – 1 ...

2012 thriller novel by Erec Stebbins The Ragnarök Conspiracy AuthorErec Stebbins[1]Cover artistNicole Sommer-LechtCountryUnited StatesLanguageEnglishGenreThriller, Political thriller, Conspiracy fiction, Spy fictionPublisherPrometheus BooksPublication dateOctober 2012Media typePrint (Paperback), ebook(Amazon Kindle, Barnes & Noble Nook)Pages350 pp (First edition)ISBN9781616147129 The Ragnarök Conspiracy is the 2012 debut thriller novel by biomedical scientist Erec Steb...

جمال إمامي جمال الدين الإمامي الخوئي معلومات شخصية الميلاد سنة 1902 خوي الوفاة سنة 1966 (63–64 سنة) باريس مواطنة الدولة البهلوية إخوة وأخوات محمد أمين الإمامي الخوئي الحياة العملية المهنة سياسي، ودبلوماسي تعديل مصدري - تعديل جمال الدين الإمامي ا�...

Kipsy redirects here. For the reputed lake monster living in the Hudson River, see Hudson River Monster. 1989 compilation album by Boy GeorgeHigh HatCompilation album by Boy GeorgeReleased1989Recorded1988Genre Pop-soul new jack swing Length51:32LabelVirginProducerGene GriffinBobby Z.Mike PelaBoy GeorgeBoy George chronology Boyfriend(1988) High Hat(1989) The Martyr Mantras(1991) High Hat is a 1989 album compiling tracks from Boy George's second and third UK and European solo albums, Te...

Room where shared broadcast equipment is located WREX-TV tech core, or CAR In broadcast facilities and television studios, a central apparatus room (CAR, pronounced C-A-R), central machine room, or central equipment room (CER), or central technical area (CTA), or rack room is where shared equipment common to all technical areas is located. Some broadcast facilities have several of these rooms. It should be air-conditioned, however low-noise specifications such as acoustical treatments are opt...

Monty Python and the Holy GrailPoster rilis layar lebar Britania RayaSutradara Terry Gilliam Terry Jones Produser Mark Forstater Michael White Ditulis oleh Monty Python Pemeran Graham Chapman John Cleese Terry Gilliam Eric Idle Terry Jones Michael Palin Penata musik Dewolfe Neil Innes SinematograferTerry BedfordPenyuntingJohn HackneyPerusahaanproduksi Python (Monty) Pictures Michael White Productions National Film Trustee Company DistributorEMI FilmsTanggal rilis 03 April 1975 (197...

Легион X «Фретензис»лат. Legio X Fretensis Надпись легиона в Иерусалиме Годы существования 41 год до н. э. — начало V века Страна Древний Рим Тип Пехота при поддержке кавалерии Численность В среднем 5000 пехоты и 300 кавалеристов Дислокация Киррус, Скифополис, Иерусалим, Айла Участ�...

Questa voce o sezione sull'argomento cani non cita le fonti necessarie o quelle presenti sono insufficienti. Puoi migliorare questa voce aggiungendo citazioni da fonti attendibili secondo le linee guida sull'uso delle fonti. Australian Shepherd(Cane da pastore australiano) Classificazione FCI - n. 342 Gruppo 1 Cani da pastore e bovari (esclusi bovari svizzeri) Sezione 1 Cani da pastore Standard n. 342 del 05/06/2009 (en fr) Nome originale Australian Shepherd Origine Stati Uniti Alt...

Melissa Etheridge Melissa Etheridge en 2008.Información personalNombre de nacimiento Melissa Lou EtheridgeNacimiento 29 de mayo de 1961 (62 años)Leavenworth, Kansas, EE. UU. Nacionalidad EstadounidenseCaracterísticas físicasOjos Avellana Cabello Rubio FamiliaCónyuge Tammy Lynn Michaels (2003-2010)Linda Wallem (2014-Presente).Pareja Julie Cypher Hijos 5EducaciónEducada en Berklee College of MusicLeavenworth High School Información profesionalOcupación Cantautora, músico, activist...

Marvel Comics fictional character Comics character Lucas BishopCover art of X-Men: The Lives and Times of Lucas Bishop #1 (March 2009) by Ariel OlivettiPublication informationPublisherMarvel ComicsFirst appearanceThe Uncanny X-Men #282 (Nov. 1991)Created byWhilce Portacio John ByrneIn-story informationSpeciesHuman mutantTeam affiliations X-Men Krakoa's Captains Marauders (Krakoa pirate crew) (Dawn of X) O*N*E Xavier's Security Enforcers Interpol The Twelve X-Treme Sanctions Executive NYPD X-T...

Fictional character in Darling in the Franxx This article is about the Darling in the Franxx character. For other uses, see 02. Fictional character Zero TwoDarling in the Franxx characterZero Two depicted in the Darling in the Franxx key visualFirst appearanceAlone and Lonesomе (2018)[1]Voiced byJapaneseHaruka Tomatsu[2]EnglishTia Ballard[3]In-universe informationAliasThe Partner Killer[4]Code:002Nine Iota[5]SpeciesKlaxosaur (Can syncretize herself int...

Method used to identify optical discs The Burst Cutting Area on an 80mm DVD A resync byte and parts of nearby zero bytes on a disc's BCA Optical discs General Optical disc Optical disc drive Optical disc authoring Authoring software Recording technologies Recording modes Packet writing Burst cutting area Optical media types Compact disc (CD): CD-DA, CD-ROM, CD-R, CD-RW, 5.1 Music Disc, Super Audio CD (SACD), Photo CD, CD Video (CDV), Video CD (VCD), Super Video CD (SVCD), CD+G, CD-Text, CD-RO...

1987 studio album by Steve Lacy & Mal WaldronSempre AmoreStudio album by Steve Lacy & Mal WaldronReleased1987RecordedFebruary 17, 1986GenreJazzLength43:14LabelSoul NoteProducerGiovanni BonandriniMal Waldron chronology Space(1986) Sempre Amore(1987) Update(1986) Steve Lacy chronology Only Monk(1987) Sempre Amore(1986) Morning Joy(1986) Sempre Amore is an album by Steve Lacy and Mal Waldron released on the Italian Soul Note label in 1987.[1] It features duo performances ...

Peta infrastruktur dan tata guna lahan di Komune Isle. = Kawasan perkotaan = Lahan subur = Padang rumput = Lahan pertanaman campuran = Hutan = Vegetasi perdu = Lahan basah = Anak sungaiIsle, Haute-Vienne merupakan sebuah komune di departemen Haute-Vienne di Prancis. Lihat pula Komune di departemen Haute-Vienne Referensi INSEE lbsKomune di departemen Haute-Vienne Aixe-sur-Vienne Ambazac Arnac-la-Poste Augne Aureil Azat-le-Ris Balledent La Bazeuge...

一場於德國斯圖加特舉行的模擬聯合國會議 模擬聯合國(英語:Model United Nations,缩写MUN)是一種學術性質活動,藉由精簡後的聯合國議規舉行模擬會議,使與會者瞭解多邊外交的過程,培養分析公民議題的能力,促進世界各地學生的交流,增進演講和辯論能力,提高组织、策划、管理、研究和写作、解决冲突、求同存异的能力[1],訓練批判性思考、團隊精神和領導才...