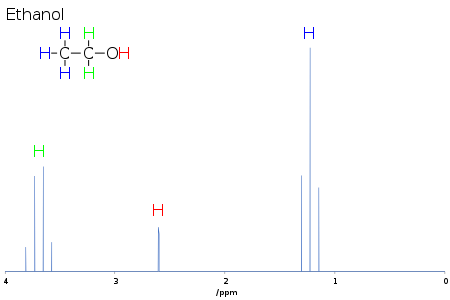
J-coupling
|
Read other articles:

Relações entre Brasil e Benin Mapa indicando localização do Brasil e do Benin. Brasil Benin As relações entre Benim e Brasil são as relações diplomáticas estabelecidas entre a República do Benim e a República Federativa do Brasil. O Brasil possui uma embaixada em Cotonou e o Benim possui uma embaixada em Brasília. O presidente Luiz Inácio Lula da Silva recebe os cumprimentos do presidente do Benim, Mathieu Kérékou, na chegada ao Palá...

Wappen Deutschlandkarte ? Hilfe zu Wappen 50.74666666666710.805555555556Koordinaten: 50° 45′ N, 10° 48′ O Basisdaten (Stand 2019) Bestandszeitraum: 1993–2019 Bundesland: Thüringen Landkreis: Ilm-Kreis Fläche: 105,89 km2 Einwohner: 9015 (31. Dez. 2017) Bevölkerungsdichte: 85 Einwohner je km2 Kfz-Kennzeichen: IK, ARN, IL Verbandsschlüssel: 16 0 70 5007 Verbandsgliederung: 7 Gemeinden Lage der ehemaligen Verwaltungsgemeinschaft im...

Медаль «За трудову доблесть»Медаль «За трудовую доблесть» Країна СРСРТип МедальВручається: робітники, колгоспники, спеціалісти народного господарства, робітники науки, культури, народної освіти, охорони здоров'я, інші громадяни СРСРСтатус не видається Нагородження Пар

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (يونيو 2019) أليس بلات معلومات شخصية الميلاد 19 ديسمبر 1963 (60 سنة) ملبورن مواطنة أستراليا الحياة العملية المهنة مغنية اللغات الإنجليزية المواقع IMDB صفحتها عل

Potret resmi, 2011 John Nichols Boozman (/ˈboʊz.mən/ BOHZ-mən; lahir 10 Desember 1950) adalah seorang politikus dan mantan optometris Amerika Serikat yang menjabat sebagai senator Amerika Serikat senior dari Arkansas, sebuah kursi dimana ia pertama kali terpilih pada 2010. Sebagai anggota Partai Republik, ia menjadi anggota DPR dari 2001 sampai 2011. Referensi Pranala luar Wikimedia Commons memiliki media mengenai John Boozman. Senator John Boozman official U.S. Senate website John Boozma...

Season of television series FrasierSeason 10DVD coverCountry of originUnited StatesNo. of episodes24ReleaseOriginal networkNBCOriginal releaseSeptember 24, 2002 (2002-09-24)[1] –May 20, 2003 (2003-05-20)Season chronology← PreviousSeason 9Next →Season 11List of episodes The tenth season of Frasier originally aired from September 24, 2002, to May 20, 2003, on NBC. The opening title screen color was changed to silver. Cast Main Kelsey Grammer as Frasier Cra...

Accidente del Beechcraft King Air de PEC Táxi Aéreo El avión accidentado en agosto de 2021, tres meses antes del accidente.Fecha 5 de noviembre de 2021Causa Vuelo controlado contra el terreno en aproximación para aterrizar; bajo investigaciónLugar 4 km al sur del aeropuerto de Piedade de Caratinga, Minas Gerais, BrasilCoordenadas 19°46′06″S 42°06′28″O / -19.7683299, -42.1077327Origen Aeropuerto Internacional de Goiânia, Goiânia, Goiás, BrasilDestino Aeropuert...

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (أبريل 2021) إضراب مزارعي البن الكولومبيين 2013 كان وقفًا لأنشطة القطاع الاقتصادي للبن الكولومبي، تم تنفيذه مع تحقيق تعبئة مختلفة في العديد من بلديات البلاد، وبالتالي تم �...

Ця стаття не містить посилань на джерела. Ви можете допомогти поліпшити цю статтю, додавши посилання на надійні (авторитетні) джерела. Матеріал без джерел може бути піддано сумніву та вилучено. (грудень 2017) Грудні плавці позначені числом 1. Грудні плавці — допомагають р�...

Цукрова промисловість — одна з найстаріших і найважливіших галузей харчової промисловості, продукція якої до 1914 року, поруч зі збіжжям, була найважливішим предметом експорту. Була одним з основних локомотивів індустріальної революції в Україні. В Україні цукор виро�...

1999 film directed by Julie Taymor TitusTheatrical release posterDirected byJulie TaymorScreenplay byJulie TaymorBased onTitus Andronicusby William ShakespeareProduced byConchita AiroldiJody AllenJulie TaymorStarring Anthony Hopkins Jessica Lange Alan Cumming Colm Feore James Frain Laura Fraser Harry Lennix Angus Macfadyen Matthew Rhys Jonathan Rhys Meyers CinematographyLuciano TovoliEdited byFrançoise BonnotMusic byElliot GoldenthalProductioncompaniesClear Blue Sky ProductionsOverseas Filmg...

Japanese anime television series Reideen The BravePromotional artwork by Tohokushinsha company勇者ライディーン(Yūsha Raidīn)GenreMechaCreated byYoshitake Suzuki Anime television seriesDirected byYoshiyuki Tomino (#01–25)Tadao Nagahama (#26–50)[1]Written byMasaki TsujiSōji YoshikawaMusic byAkihiro KomoriStudioSoeishaOriginal networkNihon Educational TelevisionOriginal run April 4, 1975 – March 26, 1976Episodes50 Anime television seriesReideen the Su...

Demographics of OttawaPopulation pyramid of Ottawa in 2021Population1,017,449 (2021)Map of Ottawa showing the francophone concentrations In 2021, the population of the city of Ottawa was 1,017,449.[1] The population of the census metropolitan area, Ottawa-Gatineau, was 1,488,307.[2] Population history Current boundariesYearPop.±%1901101,102— 1911123,417+22.1%1921152,868+23.9%1931174,056+13.9%1941206,367+18.6%1951246,298+19.3%1956287,244+16.6%1961358,4...

This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Flag of Zeeland – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this template message) The flag of Zeeland The flag of Zeeland was adopted on 14 January 1949. designed by T.A.J.W. Schorer. In the centre of the flag, the coat of arms of...

2023 FIVB Volleyball Women's Olympic Qualification Tournaments2023年女排奥运资格赛FIVBパリ五輪予選/ワールドカップバレー2023Turniej Kwalifikacyjny do Igrzysk Olimpijskich 2023Official logoTournament detailsHost nations China Japan PolandCityNingboTokyoŁódźDates16–24 SeptemberTeams24Venue(s)3 (in 3 host cities)Tournament statisticsMatches played84Attendance307,932 (3,666 per match)Official websiteFIVB Road to Paris Volleyball Qualifier�...

Ruta Estatal 905 California, Estados Unidos Estados Unidos Datos de la rutaIdentificador 905 Inauguración Octubre de 1984 por la FHWA[1]1986 por Caltrans (de la SR 117)[2]Longitud 8.964[3]AdministraciónConcesionaria CaltransOrientación • Oeste I 5 en San Diego • Este Frontera mexicana cerca de Otay MesaSiguientes rutas I 880 ← → I 980 [editar datos en Wikidata] La Ruta Estatal 905 es una carretera estata...

Cape Town BlitzPersonnelCaptainQuinton de KockCoachAshwell PrinceTeam informationCityCape Town, Western Cape, South AfricaFounded2018Dissolved2021Home groundNewlands, Cape Town T20I kit Cape Town Blitz were a franchise team of the South African Mzansi Super League (MSL) Twenty20 cricket tournament. The team was based at the Newlands Cricket Ground in Cape Town. Cape Town Blitz played in the first two editions of the MLS in 2018 and 2019, before COVID-19 delayed the competition in 2020. The si...

British online television series Extra GearGenreMotoringCreated byChris EvansDirected by Richard Down Simon Staffurth Toby Baker Presented by Rory Reid Chris Harris George Lewis Country of originUnited KingdomOriginal languageEnglishNo. of series4No. of episodes24ProductionExecutive producerMartin DanceProducers Richard Down Stephanie Fox Toby Brack Running time30 minsProduction companiesBBC / BBC StudiosBBC AmericaOriginal releaseNetwork BBC Three BBC iPlayer BBC Two (2017–2019) Release29 ...

The Martin Ennals Award for Human Rights Defenders, sometimes called the Nobel Prize for human rights,[1] is an annual prize for human rights defenders. It was created in 1993 to honour and protect individuals around the world who demonstrate exceptional courage in defending and promoting human rights. Its principal aim is to provide protection (protective publicity) to human rights defenders who are at risk by focusing international media attention on their plight, mainly through onl...

Indian scholar and writer (1942–2011) Indira GoswamiBorn(1942-11-14)14 November 1942Guwahati, Assam, British IndiaDied29 November 2011(2011-11-29) (aged 69)[1]Guwahati, Assam, India[2]Pen nameMamoni Raisom GoswamiOccupationActivist, editor, poet, professor and writerNationalityIndianPeriod1956–2011GenreAssamese literatureSubjectPlight of the dispossessed in India and abroadNotable works-The Moth Eaten Howdah of the Tusker -The Man from Chinnamasta-Pages Stained With B...