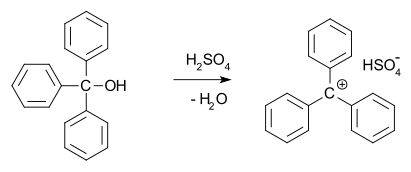
Carbocation
|
Read other articles:

Questa voce o sezione sull'argomento giornalismo è priva o carente di note e riferimenti bibliografici puntuali. Sebbene vi siano una bibliografia e/o dei collegamenti esterni, manca la contestualizzazione delle fonti con note a piè di pagina o altri riferimenti precisi che indichino puntualmente la provenienza delle informazioni. Puoi migliorare questa voce citando le fonti più precisamente. Segui i suggerimenti del progetto di riferimento. Il PopoloStato Italia Italia Ling...

Prolepsis tristis Klasifikasi ilmiah Kerajaan: Animalia Filum: Arthropoda Kelas: Insecta Ordo: Diptera Famili: Tipulidae Genus: Prolepsis Spesies: Prolepsis tristis Prolepsis tristis adalah spesies lalat yang tergolong famili Asilidae. Lalat ini juga merupakan bagian dari genus Prolepsis, ordo Diptera, kelas Insecta, filum Arthropoda, dan kingdom Animalia.[1] Lalat ini mempunyai insting predator yang agresif dan makanannya utamanya adalah serangga lain. Referensi ^ Bisby F.A., Roskov ...

Cattedrale Wesley (Kumasi) Questa è una lista delle cattedrali in Ghana. Indice 1 Cattedrali cattoliche 2 Cattedrale anglicana 3 Cattedrale metodista 4 Cattedrale ortodossa 5 Voci correlate 6 Altri progetti 7 Collegamenti esterni Cattedrali cattoliche La cattedrale di Nostra Signora di Lourdes, a Yendi Cattedrale Arcidiocesio Diocesi Località Cattedrale dello Spirito Santo Arcidiocesi di Accra Accra Cattedrale del Sacro Cuore Diocesi di Ho Ho (Ghana) Cattedrale di San Francesco Claver Dioce...

2016年夏季奥林匹克运动会马达加斯加代表團马达加斯加国旗IOC編碼MADNOC馬達加斯加奧林匹克委員會2016年夏季奥林匹克运动会(里約熱內盧)2016年8月5日至8月21日運動員6參賽項目4个大项旗手开幕式:Eliane Saholinirina(田径)[1]闭幕式:Asaramanitra Ratiarison(柔道)[2]历届奥林匹克运动会参赛记录(总结)夏季奥林匹克运动会196419681972197619801984198819921996200020042008201220162...

Este nombre sigue la onomástica japonesa; el apellido es Oda. Oda Hidenobu Información personalNombre en japonés 織田秀信 Nacimiento 1580 Provincia de Mino (Japón) Fallecimiento 24 de julio de 1605 o 13 de julio de 1605 Provincia de Mino (Japón) Nacionalidad JaponesaFamiliaPadres Oda Nobutada sin etiquetar, sin etiquetar y Shinshōni Información profesionalOcupación Samurái Conflictos Batalla de Gifu Castle [editar datos en Wikidata] Oda Hidenobu (織田秀信, Oda Hiden...

This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article uses bare URLs, which are uninformative and vulnerable to link rot. Please consider converting them to full citations to ensure the article remains verifiable and maintains a consistent citation style. Several templates and tools are available to assist in formatting, such as reFill (documentation) and Citation bot (documentatio...

Dalam tata kelola perusahaan, kodeterminasi (disebut juga kemitraan bersama atau partisipasi pekerja) adalah praktik di mana pekerja suatu perusahaan memiliki hak untuk memilih perwakilannya di dewan direksi perusahaan tersebut. Istilah ini juga merujuk pada staf yang memiliki hak yang mengikat di dalam dewan kerja mengenai permasalahan di tempat kerjanya. Praktik perwakilan di tingkat direksi tersebar luas di negara demokrasi maju.[1] Undang-Undang awal yang mewajibkan hak pilih peke...

Historic district in Massachusetts, United States United States historic placeMechanics Block Historic DistrictU.S. National Register of Historic PlacesU.S. Historic district Show map of MassachusettsShow map of the United StatesLocationLawrence, MassachusettsCoordinates42°42′34″N 71°9′17″W / 42.70944°N 71.15472°W / 42.70944; -71.15472Built1847Architectural styleItalianateNRHP reference No.73001942[1] (original)78000451 (inc...

此條目過於依赖第一手来源。 (2023年11月25日)请補充第二手及第三手來源,以改善这篇条目。 佛教 基本教義 四圣谛 八正道 十二因缘 五蘊 緣起 空性 因果 業 戒律 毗奈耶 尸羅 五戒 禪那 业处 轮回 波罗密 涅槃 真如 佛性 皈依 三寶 三法印 佛教共識宣言 修行成就/果位 佛 菩萨 辟支佛 四向四果 阿罗汉 阿那含 斯陀含 須陀洹 人物(英语:List_of_Buddhists) 释迦牟尼 十大弟子 迦�...

Mukti Arja BerlianKalakespra dr. Saryanto Informasi pribadiLahirIndonesiaAlma materSepa PK (1995)Karier militerPihak IndonesiaDinas/cabang TNI Angkatan UdaraMasa dinas1995—sekarangPangkat Marsekal Pertama TNISatuanKorps Kesehatan (Kes)Sunting kotak info • L • B Marsekal Pertama TNI dr. Mukti Arja Berlian, Sp.PD., Sp.Kp. adalah seorang perwira tinggi TNI-AU yang saat ini menjabat Kepala Lakespra dr. Saryanto. Sejak 13 September 2021 sampai dengan 26 Juni 2023 mengemba...

Hindu nationalist militant organisation Bajrang DalLogo of Bajrang DalFormation8 October 1984 (39 years ago) (1984-10-08) (Uttar Pradesh)PurposeMilitant[1] youth wing of Vishva Hindu ParishadHeadquartersNew Delhi, IndiaRegion served IndiaOfficial language HindiHeadNeeraj DoneriaParent organisationVishva Hindu ParishadAffiliationsSangh ParivarWebsitevhp.org/bajrang_dal/ Part of a series onIslamophobia Issues Airport profiling United States Conspiracy theories Counter-jiha...

Division of the National Geographic Society National Geographic Maps, founded in 1915, is the commercial map publishing division of National Geographic, part of a joint venture between The Walt Disney Company and the National Geographic Society. Initially the in-house cartographic studio for National Geographic magazine, National Geographic Maps is now responsible for the creation and distribution of commercial map products including printed wall maps and folded travel and outdoor recreation ...

American men's college basketball coach (born 1961) Karl HobbsHobbs after the 2007 Atlantic 10 tournamentCurrent positionTitleAssociate Head CoachTeamGeorgia TechConferenceACCBiographical detailsBorn (1961-08-07) August 7, 1961 (age 62)Roxbury, Massachusetts, U.S.Playing career1981–1984UConn Position(s)Point guardCoaching career (HC unless noted)1987–1993Boston University (assistant)1993–2001UConn (assistant)2001–2011George Washington2012–2016UConn (assistant)2016–2023Rutgers...

Inactive US Army formation 3rd Armored DivisionThe 3rd Armored Division's shoulder sleeve insignia.Active1941–19451947–1992Country United StatesBranch ArmyTypeArmorRoleArmored warfareSizeTypically 15,000+Nickname(s)Spearhead[1]MarchSpearhead MarchEngagementsWorld War II Normandy Northern France Rhineland Ardennes-Alsace Central Europe Gulf WarCommandersNotablecommandersMG Maurice Rose MG Gordon B. Rogers MG Creighton AbramsInsigniaFlagNATO Map Symbol 3 Military unit 3rd ...

Rethink, Thinking twice, and Thinking again redirect here. For other uses, see Rethink (disambiguation) and Think Twice (disambiguation). For the artist-activist collaborative, see Think Again. Graffiti in Antwerp combines the English word, Rethink with Chinese letter styling. Rethinking, reconsidering, or reconsideration, is the process of reviewing a decision or conclusion that has previously been made to determine whether the initial decision should be changed. Rethinking can occur immedia...

Khan of the Chagatai Khanate Ali SultanAli Sultan, as depicted by Ambrogio Lorenzetti in The Martyrdom of the Franciscans in 1342Khan of Chagatai KhanateReign1339-1342PredecessorYesun TemurSuccessorMuhammad I ibn PuladBornunknownDied1342 'Ali Khalil, also known as Ali-Sultan , was the khan (r.1339-1342)[1] of the Chagatai Khanate. He was a descendant of Qadan, son of the second Great Khan Ögedei. 'Ali attacked the ordo (palace) of Yesun Temur and usurped the throne. He was the fi...

Kidung Agung 2Ilustrasi dari ayat pertama Kidung Agung, seorang pemusik memainkan musik di hadapan raja Salomo (Rothschild Mahzor, abad ke-15 M).KitabKitab Kidung AgungKategoriKetuvimBagian Alkitab KristenPerjanjian LamaUrutan dalamKitab Kristen22← pasal 1 pasal 3 → Kidung Agung 2 (disingkat Kid 2) adalah bagian dari Kitab Kidung Agung dalam Alkitab Ibrani dan Perjanjian Lama di Alkitab Kristen.[1][2] Digubah oleh raja Salomo, putra raja Daud.[3] Teks Naska...

Artikel ini memiliki beberapa masalah. Tolong bantu memperbaikinya atau diskusikan masalah-masalah ini di halaman pembicaraannya. (Pelajari bagaimana dan kapan saat yang tepat untuk menghapus templat pesan ini) Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Gamelan Jawa – b...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Ashley Ridge High School – news · newspapers · books · scholar · JSTOR (August 2020) (Learn how and when to remove this template message) Public school in Summerville, South Carolina, United StatesAshley Ridge High SchoolAddress9800 Delemar HighwaySummerville, ...

This article is about novel by Candia McWilliam. For lands contested between England and Scotland in the 13th–17th centuries, see Debatable Lands. This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Debatable Land – news · newspapers · books · scholar · JSTOR (June 2020) (Learn how and when to remove this template message) Firs...