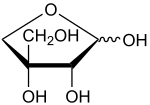
Apiose
|
| Names
|
| IUPAC name
2,3,4-Trihydroxy-3-(hydroxymethyl)butanal
|
| Other names
D-Apiose
3-C-(Hydroxymethyl)-D-glycerotetrose
Apio-β-D-furanosyl
|
| Identifiers
|
|
|
|
|
|
|
| ChEBI
|
|
| ChemSpider
|
|
| KEGG
|
|
|
|
|
| UNII
|
|
InChI=1S/C5H10O5/c6-1-5(9)2-10-4(8)3(5)7/h3-4,6-9H,1-2H2/t3-,4?,5+/m0/s1  Y YKey: ASNHGEVAWNWCRQ-LJJLCWGRSA-N  Y YInChI=1/C5H10O5/c6-1-5(9)2-10-4(8)3(5)7/h3-4,6-9H,1-2H2/t3-,4?,5+/m0/s1 Key: ASNHGEVAWNWCRQ-LJJLCWGRBE
|
|
|
| Properties
|
|
|
C5H10O5
|
| Molar mass
|
150.130 g·mol−1
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
Chemical compound
Apiose is a branched-chain sugar found as residues in galacturonans-type pectins; that occurs in parsley and many other plants. Apiose is a component of cell wall polysaccharides.[1]
Apiose 1-reductase uses D-apiitol and NAD+ to produce apiitol-apiose, NADH, and H+.
Flavone apiosyltransferase uses UDP-apiose and 5,7,4'-trihydroxyflavone 7-O-β-D-glucoside to produce UDP, 5,7,4'-trihydroxyflavone (apigenin), and 7-O-β-D-apiosyl-(1->2)-β-apiitol-glucoside.
References
External links
 The dictionary definition of apiose at Wiktionary
The dictionary definition of apiose at Wiktionary