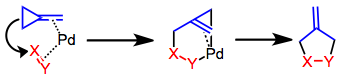
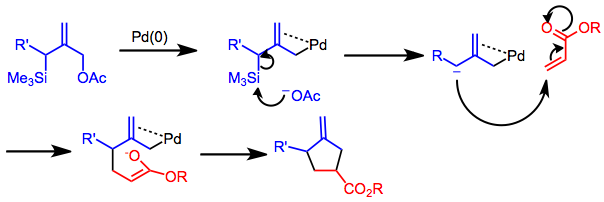
Trimethylenemethane cycloaddition
|
Read other articles:

Indian revolutionary Hero Bhupendranath Dattaশ্রী ভূপেন্দ্রনাথ দত্তSri Bhupendranath DattaBornBhupendranath Datta(1880-09-04)4 September 1880Calcutta, Bengal Presidency, British IndiaDied25 December 1961(1961-12-25) (aged 81)Calcutta, West Bengal, IndiaNationalityIndianEducationNew York University Brown University University of HamburgOccupation(s)Revolutionary Freedom Fighter Biological AnthropologistKnown forBeing a RevolutionaryNotable work...

此條目介紹的是國道二號的大園交流道。关于台61線同名之交流道,请见「大園交流道 (台61線)」。 大園交流道Dayuan Interchange 1 大園 2 大園所在地臺灣桃園市大園區埔心地理坐标25°03′31.7″N 121°12′47.8″E / 25.058806°N 121.213278°E / 25.058806; 121.213278坐标:25°03′31.7″N 121°12′47.8″E / 25.058806°N 121.213278°E / 25.058806; 121.213...

この記事には複数の問題があります。改善やノートページでの議論にご協力ください。 出典がまったく示されていないか不十分です。内容に関する文献や情報源が必要です。(2021年3月) 古い情報を更新する必要があります。(2021年3月)出典検索?: Netflix – ニュース · 書籍 · スカラー · CiNii · J-STAGE · NDL · dlib.jp · ジャパンサー�...

40-й окремий мотопіхотний батальйон Нарукавний знак батальйонуНа службі 15 травень 2014 — квітень 2015Країна УкраїнаВид Сухопутні військаТип Механізовані військаЧисельність БатальйонУ складі 17 ОТБрПункт базування Дніпропетровська область, Кривий РігРічниці 15

Halaman ini berisi artikel tentang the men's team. Untuk the women's team, lihat Mongolia women's national football team. MongoliaJulukanХөх Чононууд (Khökh Chononuud) (Blue Wolves) Чингис Хаан (Tchingis Khaan) (Genghis Khan)AsosiasiFederasi Sepak Bola Mongolia (MFF)KonfederasiAFC (Asia)Sub-konfederasiEAFF (Asia Timur)PelatihShuichi MaseKaptenTsend-Ayush KhurelbaatarPenampilan terbanyakGaridmagnai Bayasgalangiin Lümbengarav Donorov Tsedenbal Norjmoogiin (35)Pencetak gol...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Christy Cabanne – news · newspapers · books · scholar · JSTOR (May 2019) (Learn how and when to remove this template message) American film director, screenwriter and actor Christy CabanneCabanne c. 1917BornWilliam Christy Cabanne(1888-04-16)April 16, 1888St. L...
Santé et bien-être Pour un article plus général, voir Objectifs de développement durable. Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. La mise en forme de cet article est à améliorer (mars 2021). La mise en forme du texte ne suit pas les recommandations de Wikipédia : il faut le « wikifier ». Comment faire ? Les points d'amélioration suivants sont les cas les plus fréquents. Le détail des points à revoir est peut-être pré...

Patung dada marmer 'Matidia 1' s.119 M Gaya rambut di Romawi berubah-ubah, dan terutama pada Zaman Kekaiaran Romawi, terdapat sejumlah cara berbeda untuk merias rambut. Seperti halnya busana, terdapat beberapa gaya rambut yang dibatasi untuk orang-orang tertentu dalam masyarakat kuno. Sehingga, gaya rambut merupakan kekhasan yang membolehkan para cendekiawan saat ini untuk membuat kronologi seni rupa dan potret Romawi; mereka dapat menanggali gambar-gambar permaisuri pada koin-koin, atau meng...

Канівський музей народного декоративного мистецтва 49°45′06″ пн. ш. 31°27′33″ сх. д. / 49.751857247397296646° пн. ш. 31.45944104775449901° сх. д. / 49.751857247397296646; 31.45944104775449901Координати: 49°45′06″ пн. ш. 31°27′33″ сх. д. / 49.751857247397296646° пн. ш. 31.459441047754499...

1998 single by Dave Matthews Band Stay (Wasting Time)Single by Dave Matthews Bandfrom the album Before These Crowded Streets ReleasedJune 28, 1998GenreRockLength5:35 (Album version)4:34 (Album/remix edit)LabelRCASongwriter(s)Matthews, Lessard, LeRoi MooreProducer(s)Steve LillywhiteDave Matthews Band singles chronology Don't Drink the Water (1998) Stay (Wasting Time) (1998) Crush (1998) Stay (Wasting Time) is a song by Dave Matthews Band, released as the second single off their album Before Th...

Penghargaan Film Nasional (India) ke-28Penghargaan Film Nasional ke-28Dianugerahkan untukFilm terbaik dari sinema India pada 1980DipersembahkanolehDirektorat Festival FilmPenganugerahanApril 1981 (1981-04)Situs web resmidff.nic.inSorotanFilm Cerita TerbaikAkaler ShandhaneyPenghargaan terbanyakAkaler Shandhaney dan Oppol (4) ← ke-27 Penghargaan Film Nasional (India) ke-29 → Penghargaan Film Nasional ke-28, yang dipersembahkan oleh Direktorat Festival Film, sebuah org...

American architect William Robert WareBorn27 May 1832Died9 June 1915 (aged 83)OccupationArt historianEmployerMassachusetts Institute of Technology Department of Architecture[edit on Wikidata] William Robert Ware (May 27, 1832 – June 9, 1915), born in Cambridge, Massachusetts into a family of the Unitarian clergy, was an American architect,[1] author, and founder of two important American architectural schools. He received his own professional education at Milton Academy, Har...

American astronomer (1868–1921) Henrietta Swan LeavittBorn(1868-07-04)July 4, 1868Lancaster, Massachusetts, U.S.DiedDecember 12, 1921(1921-12-12) (aged 53)Cambridge, Massachusetts, U.S.EducationOberlin CollegeHarvard University (BS)Known forLeavitt's law: the period-luminosity relationship for Cepheid variablesScientific careerFieldsAstronomyInstitutionsHarvard University Henrietta Swan Leavitt (/ˈlɛvɪt/; July 4, 1868 – December 12, 1921[1]) was an American astronomer...

Royal-class cruise ship Majestic Princess Majestic Princess in Fremantle, 2023 History United Kingdom NameMajestic Princess Owner Carnival Corporation & plc OperatorPrincess Cruises Port of registryLondon, United Kingdom Ordered30 July 2014[4] BuilderFincantieri (Monfalcone, Italy)[4] Yard number6232[2] Laid down10 July 2015[5] Launched8 February 2016[1] Sponsored byYao Ming and Ye Li[6] Christened9 July 2017[6] Acquired30 Marc...

Private university in New York City, New York, U.S. This article is about the private institution founded in 1831. For other and similar uses, see University of New York. NYU redirects here. For the district in Japan, see Nyū District, Fukui. For the Elfen Lied character, see List of Elfen Lied characters § Nyu. New York UniversityLatin: Universitas Neo EboracensisFormer nameUniversity of the City of New-York (1831–1896)MottoPerstare et praestare (Latin)Motto in EnglishTo persevere a...

Heiligdom van Asklepios in Epidaurus Werelderfgoed cultuur Land Griekenland Coördinaten 37° 36′ NB, 23° 5′ OL UNESCO-regio Europa en Noord-Amerika Criteria i, ii, iii, iv, vi Inschrijvingsverloop UNESCO-volgnr. 491 Inschrijving 1988 (12e sessie) Kaart UNESCO-werelderfgoedlijst Epidaurus (Oudgrieks: Επίδαυρος, Epídauros) is de naam van een oud-Griekse stad op het schiereiland Argolis, beroemd om het nabijgelegen Grieks heiligdom van Asklepios, halfgod van de gene...

NFL seasonal playoff games 2000–01 NFL playoffsDatesDecember 30, 2000–January 28, 2001Season2000Teams12Games played11Super Bowl XXXV siteRaymond James StadiumTampa, FloridaDefending championsSt. Louis RamsChampionsBaltimore RavensRunners-upNew York GiantsConferencerunners-upMinnesota VikingsOakland Raiders NFL playoffs ← 1999–2000 2001–02 → The National Football League playoffs for the 2000 season began on December 30, 2000. The postseason tournament concluded with the Baltimore R...

Untuk kegunaan lain, lihat Harta karun (disambiguasi). Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Harta karun – berita · surat kabar · buku · cendekiawan · JSTOR Harta karun adalah sejumlah besar harta atau kekayaan lain yang tersembunyi, maupun ...

В Википедии есть статьи о других людях с фамилией Янин. Костя Янин Имя при рождении Константин Александрович Янин Дата рождения 15 июня 1931(1931-06-15) Дата смерти 5 сентября 1943(1943-09-05) (12 лет) Место смерти Ямпольский район, Сумская область, Украинская ССР, СССР Страна СССР...

中国共产党 历史 - 章程 - 组织 - 领导 最高领导人 中央委员会主席 中央委员会副主席 中央委员会总书记 中央政治局常务委员会委员 中央委员会秘书长 中央书记处总书记 标志和制度 党旗、党徽、党报、党刊 领导集体、干部制度、民主集中制 纪律检查机关 指导思想 马克思列宁主义 毛泽东思想 中国特色社会主义理论体系 邓小平理论 “三个代表”重要思想 科学发展观 习近...