Silicon tetrachloride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Animated television series For other uses, see Dragon Prince (disambiguation). The Dragon PrinceAlso known asThe Dragon Prince: Mystery of Aaravos (season 4–present)GenreFantasyActionAdventureComedy dramaCreated byAaron EhaszJustin RichmondWritten byAaron EhaszJustin RichmondDevon GiehlIain HendryNeil MukhopadhyayDirected byVillads SpangsbergGiancarlo VolpeVoices ofJack DeSenaPaula BurrowsSasha RojenJason SimpsonRacquel BelmonteJesse InocallaComposerFrederik WiedmannCountry of originUnited ...
هذه المقالة بحاجة لصندوق معلومات. فضلًا ساعد في تحسين هذه المقالة بإضافة صندوق معلومات مخصص إليها. هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (مايو 2021) الساحل الغربي لأمريكا الشمالية يُعتقد أن التاريخ البشري للس

Untuk kegunaan lain dari Sodom, lihat sodom (disambiguasi) Lukisan Terbakarnya Sodom dan Gomora oleh Jacob de Wet II, 1680 Sodom (bahasa Arab: سدوم Sadūm,bahasa Ibrani: סְדוֹם, Modern Sədom Tiberias Səḏôm, Bahasa Yunani: Σόδομα Sódoma) dan Amora (bahasa Arab: عمورة ʿAmūrah, bahasa Ibrani: עֲמוֹרָה, Modern ʿAmora Tiberias Ġəmôrāh Ămôrāh, bahasa Yunani: Γόμορρα Gómorra) atau Gomora (bahasa Ibrani: עֲמוֹרָה, Modern...

Jagoan InstanBersiap hadapi Jagoan Instan!!!Sutradara Fajar Bustomi Produser Chand Parwez Servia Fiaz Servia Ditulis oleh Musfar Yasin PemeranKemal PaleviKevin JulioAnisa RahmaDede Yusuf Meriam BellinaAlexa KeyJovial da LopezAndovi da LopezPenata musikAndhika TriyadiSinematograferJoel Fadly ZolaPenyuntingGanda HartadiPerusahaanproduksiStarvision PlusDistributorStarvision PlusTanggal rilis18 Februari 2016Durasi96 menitNegara Indonesia Bahasa Indonesia Jagoan Instan adalah film aksi komed...

Рерайтинг — термін, який має кілька значень. Ця сторінка значень містить посилання на статті про кожне з них.Якщо ви потрапили сюди за внутрішнім посиланням, будь ласка, поверніться та виправте його так, щоб воно вказувало безпосередньо на потрібну статтю.@ пошук посилань

2003 video gameThe Omega StoneDeveloper(s)Omni World Studios[a]Publisher(s)DreamCatcher InteractiveDirector(s)Jeffrey S. ToblerKaren E. ToblerProgrammer(s)Thomas CartonMichael CreightonJeffrey S. ToblerComposer(s)Jeffrey S. ToblerPlatform(s)Windows, Mac OS XReleaseMarch 18, 2003 (Win)[1]March 9, 2004 (Mac)[2]Genre(s)AdventureMode(s)Singleplayer The Omega Stone is a Microsoft Windows puzzle adventure game developed by American studio Omni World Studios. It was the seque...

Esta página cita fontes, mas que não cobrem todo o conteúdo. Ajude a inserir referências. Conteúdo não verificável pode ser removido.—Encontre fontes: ABW • CAPES • Google (N • L • A) (Janeiro de 2023) SÉCULOS: Século XIX — Século XX — Século XXI DÉCADAS: 1930 • 1940 • 1950 • 1960 • 1970 • 1980 • 1990 • 2000 • 2010 • 2020 • 2030 ANOS: 1978 • 1979 • 1980 • 1981 • 1982 •&#...

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (مارس 2020) فرانسيس شيهي سكيفينغتون معلومات شخصية اسم الولادة (بالإنجليزية: Francis Joseph Christopher Skeffington) الميلاد 23 ديسمبر 1878[1] الوفاة 26 أبريل 1916 (37 سنة) مواطنة الم
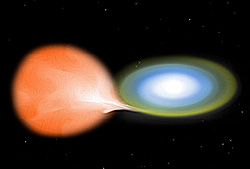
Nuclear explosion in a white dwarf star For other uses, see Nova (disambiguation), Novas (disambiguation), and Novae (disambiguation). Not to be confused with luminous red nova, supernova, kilonova, or micronova. Artist's conception of a white dwarf, right, accreting hydrogen from the Roche lobe of its larger companion star A nova (pl.: novae or novas) is a transient astronomical event that causes the sudden appearance of a bright, apparently new star (hence the name nova, which is Latin for ...

The Best of Philip K. Dick Cover of the first edition.AuthorPhilip K. DickCover artistVincent Di FateCountryUnited StatesLanguageEnglishSeriesBallantine's Classic Library of Science FictionGenreScience fictionPublisherDel Rey BooksPublication date1977Media typePrint (paperback)Pagesxiv, 450ISBN0-345-25359-0OCLC2645491Dewey Decimal813/.5/4LC ClassPS3554.I3 B4Preceded byThe Best of C. M. Kornbluth Followed byThe Best of Fredric Brown The Best of Philip K. Dic...

العلاقات الصينية اللاوسية الصين لاوس الصين لاوس تعديل مصدري - تعديل العلاقات الصينية اللاوسية هي العلاقات الثنائية التي تجمع بين الصين ولاوس.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين: وجه المقارنة الصين لاوس المساح

Iranian ayatollah AyatollahMohsen ArakiMember of Expediency Discernment CouncilIncumbentAssumed office 20 September 2022Appointed byAli KhameneiChairmanSadeq LarijaniMember of the Assembly of ExpertsIncumbentAssumed office 24 May 2016ConstituencyMarkazi ProvinceMajority242,146In office21 February 1991 – 20 February 2007ConstituencyKhuzestan Province Personal detailsBorn1956Najaf, IraqWebsiteOfficial website Mohsen Araki (Persian: محسن اراکی; Arabic: محسن الأ...

Genus of spiders Unicorn Male Unicorn sikus, viewed from above (legs omitted). Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Arthropoda Subphylum: Chelicerata Class: Arachnida Order: Araneae Infraorder: Araneomorphae Family: Oonopidae Genus: UnicornPlatnick & Brescovit, 1995 Type species Unicorn catleyiPlatnick & Brescovit, 1995 Species 7, see text Unicorn (one horn, in Latin) is a genus of goblin spiders (family Oonopidae) from South America, co...

This article may need to be rewritten to comply with Wikipedia's quality standards, as outdated. You can help. The talk page may contain suggestions. (December 2015) Hove FestivalGenreIndie, pop, metal, hip hop, electronic musicDates2 – 5 July (2013)Location(s)Tromøya, Arendal, NorwayCoordinates58°26′33″N 8°50′50″E / 58.4426°N 8.8471°E / 58.4426; 8.8471Years active2007 – 2014Founded byToffen Gunnufsen (2007 – 2008) Melvin Benn 2009 –Websitewww.hove...

Dalam nama yang mengikuti kebiasaan penamaan Slavia Timur ini, patronimiknya adalah Vasilevna dan nama keluarganya adalah Kostenko. Natalya KostenkoНаталья КостенкоDeputi Duma Negara ke-8PetahanaMulai menjabat 12 Oktober 2021Deputi Duma Negara ke-7Masa jabatan5 Oktober 2016 – 12 Oktober 2021 Informasi pribadiLahir09 Agustus 1980 (umur 43)Malotenginskaya, Krai Krasnodar, Rusia, Uni SovietPartai politikRusia BersatuAlma materUniversitas Negeri KubanSunting...

Este artigo ou seção pode conter informações desatualizadas. Se tem conhecimento sobre o tema abordado, edite a página e inclua as informações mais recentes, citando fontes fiáveis e independentes. —Encontre fontes: ABW • CAPES • Google (N • L • A) (Outubro de 2022) As referências deste artigo necessitam de formatação. Por favor, utilize fontes apropriadas contendo título, autor e data para que o verbete permaneça ...

This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (August 2020) (Learn how and when to remove this template message) Sarojini Yogeswaran14th Mayor of JaffnaIn office1998–1998Succeeded byPon SivapalanMember of Jaffna Municipal CouncilIn office1998–1998 Personal detailsBornSarojini PonnambalamDied(1998-05-17)17 May 1998Jaffna, Sri LankaPolitical partyTamil Uni...

Pierre-François Bouchard menemukan Batu Rosetta. Pierre-François Bouchard (29 April 1771 – 5 Agustus 1822) adalah perwira zeni Angkatan Darat Prancis yang terkenal karena menemukan Batu Rosetta, temuan arkeologis penting yang memungkinkan sastra Mesir Kuno dapat dipahami orang untuk pertama kalinya sesudah lebih dari satu milenium. Daftar pustaka (Prancis) La chute d'El-Arich, décembre 1799 : Journal historique du Capitaine Bouchard, preface and notes by Gaston Wiet, �...

AirportOttumwa Regional AirportIATA: OTMICAO: KOTMFAA LID: OTMSummaryAirport typePublicOwnerCity of OttumwaServesOttumwa, IowaElevation AMSL845 ft / 258 mCoordinates41°06′24″N 092°26′53″W / 41.10667°N 92.44806°W / 41.10667; -92.44806MapOTMShow map of IowaOTMShow map of the United StatesRunways Direction Length Surface ft m 13/31 5,885 1,794 Asphalt/concrete 4/22 4,600 1,402 Asphalt StatisticsAircraft operations (2015)16,450Based aircraft (201...

Stadion Miejski w Elblągu(Stadion Olimpii Elbląg) Trybuna stadionu po modernizacji w 2011 roku Państwo Polska Województwo warmińsko-mazurskie Miejscowość Elbląg Adres ul. Agrykola 882-300 Elbląg Data budowy ?, 2010/2011 (modernizacja) Data otwarcia 1951/1952 Właściciel Urząd Miejski w Elblągu Klub Olimpia Elbląg Pojemność stadionu 3000 miejsc 125 (miejsc dla V.I.P-ów) 750 (miejsc dla gości)[1] Rekordowa frekwencja 12 000 widzów29 sierpnia 1976,O. Elbląg – Z. ...



