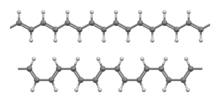
Polyacetylene
| |||||||||||||||||||||||||||||||||||||
Read other articles:

Augusto Castellani Augusto Castellani (Roma, 10 gennaio 1829 ŌĆō Roma, 23 gennaio 1914) ├© stato un orafo italiano, collezionista e mercante antiquario attivo a Roma, ma con una grande clientela internazionale. Indice 1 La famiglia di orafi 2 Augusto, il collezionista 3 Scritti 4 Albero genealogico[4][5] 5 Note 6 Bibliografia 7 Voci correlate 8 Altri progetti 9 Collegamenti esterni La famiglia di orafi Il capostipite della famiglia Castellani fu Fortunato Pio (1794-1865), oraf...

For other uses, see Robotnik (1983ŌĆō1990) and Robotnik (disambiguation). RobotnikRobotnik, front page of issue from 28 October 1931.Typevaried, later daily newspaperFounded1894Political alignmentsocialistLanguagePolish languageCeased publication1939 Robotnik (Polish pronunciation: [r╔ö╦łb╔öt╔▓ik]; The Worker) was the bibu┼éa (underground) newspaper published by the Polish Socialist Party (PPS), and distributed in most major cities and towns in Poland during the Partitions.[1]...

Maxfield Parrish Informaci├│n personalNacimiento 25 de julio de 1870 Filadelfia (Estados Unidos) Fallecimiento 30 de marzo de 1966 (95 a├▒os)Nuevo Hampshire (Estados Unidos) o Plainfield (Estados Unidos) Nacionalidad EstadounidenseFamiliaPadre Stephen Parrish Educaci├│nEducado en Academia de Bellas Artes de PensilvaniaHaverford CollegeThe Haverford School Alumno de Howard Pyle Informaci├│n profesionalOcupaci├│n Pintor, ilustrador y dise├▒ador G├®neros Pintura mitol├│gica, arte figurativo y es...

ą£ąĄą╗ąĄąĮč¢ čüčéą░ąĮčåč¢čÅąĀąŠąĘčéą░čłčāą▓ą░ąĮąĮčÅąĀąŠąĘčéą░čłčāą▓ą░ąĮąĮčÅ ąĮą░ąĘąĄą╝ąĮą░ąÜąŠąŠčĆą┤ąĖąĮą░čéąĖ 50┬░53ŌĆ▓21ŌĆ│ ą┐ąĮ. čł. 28┬░52ŌĆ▓08ŌĆ│ čüčģ. ą┤. / 50.88917710002777284┬░ ą┐ąĮ. čł. 28.869155400027779024┬░ čüčģ. ą┤. / 50.88917710002777284; 28.869155400027779024ąÜąŠąŠčĆą┤ąĖąĮą░čéąĖ: 50┬░53ŌĆ▓21ŌĆ│ ą┐ąĮ. čł. 28┬░52ŌĆ▓08ŌĆ│ čüčģ. ą┤. / 50.88917710002777284┬░ ą┐ąĮ. čł. 28.86...

ķĆāŃüÆŃéŗŃü»µüźŃüĀŃüīÕĮ╣Ńü½ń½ŗŃüżSz├®gyen a fut├Īs, de hasznos. ŃéĖŃāŻŃā│Ńā½ Ńā®Ńā¢Ńé│ŃāĪ µ╝½ńö╗ õĮ£ĶĆģ µĄĘķćÄŃüżŃü¬Ńü┐ Õć║ńēłńżŠ Ķ¼øĶ½ćńżŠ µÄ▓Ķ╝ēĶ¬ī Kiss Ńā¼Ńā╝ŃāÖŃā½ KC Kiss ńÖ║ĶĪ©µ£¤ķ¢ō 2012Õ╣┤22ÕÅĘ - 2017Õ╣┤2µ£łÕÅĘ2019Õ╣┤3µ£łÕÅĘ - 2020Õ╣┤4µ£łÕÅĘ ÕĘ╗µĢ░ Õģ©11ÕĘ╗ Ķ®▒µĢ░ Õģ©54Ķ®▒ ŃāēŃā®Ńā× ÕĤõĮ£ µĄĘķćÄŃüżŃü¬Ńü┐ Ķäܵ£¼ ķćĵ£©õ║£ń┤ĆÕŁÉ µ╝öÕć║ ķćæÕŁÉµ¢ćń┤ĆŃĆüÕ£¤õ║ĢĶŻĢµ│░ŃĆüń¤│õ║ĢÕ║ʵÖ┤ ķ¤│µźĮ µ£½Õ╗ŻÕüźõĖĆķāÄŃĆüMAYUKO ĶŻĮõĮ£ TBSTBS SPARKLE’╝łŃé╣ŃāÜŃéĘŃāŻŃā½’╝ē µöŠķĆüÕ▒Ć TBSń│╗ÕłŚ µöŠķĆüµ£¤ķ¢ō ķĆŻ

ž¦┘äž│┘Ŗž¦žŁž® ┘ü┘Ŗ ž¦┘äž©žŁž▒┘Ŗ┘å ž¬ž╣ž» žŻ┘ć┘ģ ┘ģžĄž¦ž»ž▒ ž¦┘äž»ž«┘ä ┘ü┘Ŗ ž¦┘ä┘ģ┘ģ┘ä┘āž® žź┘ä┘ē ž¼ž¦┘åž© ž¦┘䞥┘垦ž╣ž®žī ┘łž¬ž»ž╣┘ģ ž¦┘䞦┘鞬žĄž¦ž» ž¦┘äž©žŁž▒┘Ŗ┘å┘Ŗ ž©┘é┘łž®. ┘łž¬ž¼ž░ž© ž¦┘äž©žŁž▒┘Ŗ┘å ž│┘å┘ł┘Ŗž¦ žŻ┘āž½ž▒ ┘ģ┘å ┘ģ┘ä┘Ŗ┘ł┘å┘Ŗ ž▓ž¦ž”ž▒ ž¦┘ä┘åž│ž©ž® ž¦┘ä┘āž©ž▒┘ē ┘ģ┘å ž¦┘äž│┘Ŗž¦žŁ ┘ć┘ģ ┘ģ┘å ž»┘ł┘ä ž¦┘äž«┘ä┘Ŗž¼ ž¦┘äž╣ž▒ž©┘Ŗžī ┘ł┘ŖžŻž¬┘Ŗ ┘ü┘Ŗ ž¦┘ä┘ģž▒ž¬ž©ž® ž¦┘䞯┘ł┘ä┘ē ž¦┘äž│┘Ŗž¦žŁ ž¦┘äž│ž╣┘łž»┘Ŗ┘Ŗ┘åžī ┘łž¦┘ä┘Ć ž¦┘ä┘ā┘ł┘Ŗž¬┘Ŗ┘Ŗ┘å ž½┘ģ ž¦┘äž╣┘ģž¦┘å┘Ŗ┘Ŗ┘åžī ┘ü┘Ć ž¦┘äžź┘ģž¦ž▒ž¦ž¬┘Ŗ┘...

The prompt book, also called transcript, the bible or sometimes simply the book, is the copy of a production script that contains the information necessary to create a theatrical production from the ground up. It is a compilation of all blocking, business, light, speech and sound cues, lists of properties, drawings of the set, contact information for the cast and crew, and any other relevant information that might be necessary to help the production run smoothly.[citation needed] In m...

Pushing of heterosexual norms onto LGBT culture Part of a series onLGBT topics LesbianGayBisexualTransgender Sexual orientation and gender Aromanticism Asexuality Gray asexuality Biology Bisexuality Pansexuality Demographics Environment Gender fluidity Gender identity Gender role Gender variance Homosexuality Intersex Non-heterosexual Non-binary gender Queer Queer heterosexuality Questioning Sexual identity SexŌĆōgender distinction Trans man Trans woman Tra...

2010 video game 2010 video gameDJ Hero 2DJ Hero 2 cover artDeveloper(s)FreeStyleGamesPublisher(s)ActivisionSeriesHeroPlatform(s)PlayStation 3, Wii, Xbox 360ReleaseNA: October 19, 2010AU: October 19, 2010EU: October 22, 2010[1]Genre(s)Music, RhythmMode(s)Single-player, local & online multiplayer DJ Hero 2 is a rhythm video game and a sequel to DJ Hero, a spinoff of the Guitar Hero series. DJ Hero 2 uses a special turntable-controller, the same as introduced in DJ Hero, to simulate ...

A seguinte lista apresenta, por ordem alfab├®tica do ├║ltimo nome, economistas not├│rios que receberam ou o pr├®mio da Banca da Su├®cia em mem├│ria de Alfred Nobel ou um pr├®mio internacionalmente reconhecido, ou porque s├Żo autores de conceitos, obras te├│ricas e pr├Īticas fundamentais na hist├│ria do pensamento econ├┤mico: ├Źndice: Ō¢▓ ┬Ę A B C D E F G H I J K L M N O P Q R S T U V W X Y Z A Nome Nasc. Morte Nacionalidade Coment├Īrios Acemoglu, Daron 1967...

Naval AcademyAccademia NavaleTypeNaval AcademyEstablishedNovember 6, 1881Officer in chargeRear admiral Flavio BiaggiLocationLivorno, Tuscany, Italy43┬░31ŌĆ▓37ŌĆ│N 10┬░18ŌĆ▓29ŌĆ│E / 43.527┬░N 10.308┬░E / 43.527; 10.308WebsiteAcademy website A view from the waterfront of L'Accademia Navale The Italian Naval Academy (Italian: Accademia Navale) is a coeducational military university in Livorno, which is responsible for the technical training of military officers of the Ita...
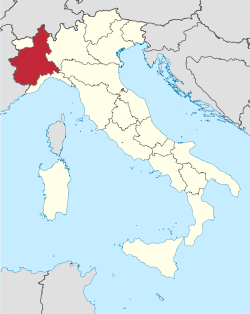
Region of Italy, part of Northwest Italy For other uses, see Piedmont (disambiguation). You can help expand this article with text translated from the corresponding article in Italian. (March 2020) Click [show] for important translation instructions. Machine translation, like DeepL or Google Translate, is a useful starting point for translations, but translators must revise errors as necessary and confirm that the translation is accurate, rather than simply copy-pasting machine-translate...

Joe Henry Informaci├│n personalNombre de nacimiento Joseph Lee HenryNacimiento 2 de diciembre de 1960 (63 a├▒os)Charlotte, Carolina del Norte, Estados UnidosCharlotte (Estados Unidos) Nacionalidad EstadounidenseFamiliaC├│nyuge Melanie CicconeEducaci├│nEducado en Universidad de M├ŁchiganRochester Adams High School Informaci├│n profesionalOcupaci├│n Cantautor, productorA├▒os activo desde 1986G├®neros Americana, acoustic blues y jazzInstrumento GuitarraDiscogr├Īfica Anti- RecordsArtis...

Indian Army General This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) An editor has performed a search and found that sufficient sources exist to establish the subject's notability. These sources can be used to expand the article and may be described in edit summaries or found on the talk page. The article may include original research, or omit significant information about the subject. Ple...

Utica Avenue Estaci├│n del Metro de Nueva YorkUbicaci├│nCoordenadas 40┬░40ŌĆ▓45ŌĆ│N 73┬░55ŌĆ▓45ŌĆ│O / 40.6792, -73.9291Direcci├│n Utica Avenue & Fulton StreetBrooklyn, NY 11233Localidad Bedford-Stuyvesant, BrooklynDatos de la estaci├│nC├│digo 181Inauguraci├│n 9 de abril de 1936Pasajeros 4.236 millones[1][2]Servicios A (altas horas de la noche) C (siempre excepto altas horas de la noche)Conexiones New York City Bus: B25, B46N.┬║ de andenes 2 Plataf...

Dutch country pop artist (born 1953) This article's use of external links may not follow Wikipedia's policies or guidelines. Please improve this article by removing excessive or inappropriate external links, and converting useful links where appropriate into footnote references. (December 2023) (Learn how and when to remove this template message) This article contains too many pictures for its overall length. Relevant discussion may be found on the talk page. Please improve this article by re...

Ńā¬ŃāüŃāŻŃā╝ŃāēŃā╗ŃāŁŃéĖŃāŻŃā╝Ńé╣Richard Rodgers Ńé╗Ńā│ŃāłŃā╗ŃéĖŃé¦Ńā╝ŃāĀŃé║ÕŖćÕĀ┤Ńü½Ńü”’╝ł1948Õ╣┤’╝ēÕ¤║µ£¼µāģÕĀ▒Õć║ńö¤ÕÉŹ Richard Charles Rodgersńö¤Ķ¬Ģ (1902-06-28) 1902Õ╣┤6µ£ł28µŚźÕć║Ķ║½Õ£░ ŃéóŃāĪŃā¬Ńé½ÕÉłĶĪåÕøĮ ŃāŗŃāźŃā╝Ńā©Ńā╝Ńé»ÕĘ×ŃāŗŃāźŃā╝Ńā©Ńā╝Ńé»ÕĖéŃé»ŃéżŃā╝Ńā│Ńé║Õī║µŁ╗µ▓Ī (1979-12-30) 1979Õ╣┤12µ£ł30µŚź’╝ł77µŁ│µ▓Ī’╝ēŃéĖŃāŻŃā│Ńā½ Ńā¤ŃāźŃā╝ŃéĖŃé½Ńā½ Ńā¬ŃāüŃāŻŃā╝ŃāēŃā╗ŃāüŃāŻŃā╝Ńā½Ńé║Ńā╗ŃāŁŃéĖŃāŻŃā╝Ńé╣’╝łRichard Charles Rodgers, 1902Õ╣┤6µ£ł28µŚź - 1979Õ╣┤12µ£ł30µŚź’╝ēŃü»ŃĆüŃéóŃāĪŃā¬Ńé½ÕÉłĶ...

Jurgis ┼Āaulys Jurgis ┼Āaulys (pengucapan Lituania: [╦łj╩Ŗr╔Ī╩▓╔¬s ╩āau╦łl╩▓├«╦És] simakŌōś; 1879ŌĆō1948) adalah seorang ekonom, diplomat dan politikus asal Lituania, dan salah satu dari dua puluh penandatangan untuk Undang-Undang Kemerdekaan Lituania tahun 1918. ┼Āaulys masuk sekolah menengah di Palanga dan masuk Seminari Teologi Kaunas. Referensi ┼Āaulys, Jurgis. Encyclopedia Lituanica V: 78-79. (1970-1978). Ed. Simas Su┼Šied─Ślis. Boston, Massachusetts: Juozas Kapo─Źius. LCC 74-1...

õ║║µ░ŚĶĆģŃü¦ŃüäŃüōŃüå! > ĶŖĖĶāĮõ║║µĀ╝õ╗śŃüæŃāüŃé¦ŃāāŃé» Oh!Ńü®ŃéäķĪöŃéĄŃā¤ŃāāŃāł > ĶŖĖĶāĮõ║║µĀ╝õ╗śŃüæŃāüŃé¦ŃāāŃé» ŃāåŃā¼ŃāōńĢ¬ńĄäŃā╗õĖŁńČÖÕåģŃü¦Ńü«ÕÉäń©«µāģÕĀ▒’╝łńĄéõ║åŃüŚŃü¤ńĢ¬ńĄäŃā╗õĖŁńČÖŃéÆÕɽŃü┐ŃüŠŃüÖ’╝ēŃü»ŃĆüDVDŃéäBlu-rayŃü¬Ńü®Ńü¦Ńü«Ķ▓®ÕŻ▓ŃéäÕģ¼Õ╝ÅŃü¬ŃāŹŃāāŃāłķģŹõ┐ĪŃĆüŃüŠŃü¤Ńü»õ┐ĪķĀ╝Ńü¦ŃüŹŃéŗń┤ÖÕ¬ÆõĮōŃüŠŃü¤Ńü»Ńé”Ńé¦Ńā¢Õ¬ÆõĮōŃüīń┤╣õ╗ŗŃüÖŃéŗŃüŠŃü¦ŃĆüÕć║ÕģĖŃü©ŃüŚŃü”ńö©ŃüäŃü¬ŃüäŃü¦õĖŗŃüĢŃüäŃĆ鵿£Ķ©╝ÕÅ»ĶāĮµĆ¦Ńü½Õ¤║ŃüźŃüŹķÖżÕÄ╗ŃüĢŃéīŃéŗÕĀ┤ÕÉłŃüīŃüéŃéŖŃüŠŃüÖŃĆé ŃüōŃü«Ķ©śõ║ŗŃü»µ...

Questa voce sull'argomento film drammatici ├© solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. La torre del piacere / La torre di NesleTitolo originaleLa Tour de Nesle Lingua originalefrancese Paese di produzioneFrancia, Italia Anno1955 Durata120 min Rapporto1,37:1 Generedrammatico, storico RegiaAbel Gance Soggettoda una commedia di Alexandre Dumas padre SceneggiaturaAbel Gance ProduttoreFilmcostellazione, Les Films Fernand Rivers Produttore esecu...