Inner sphere complex
|
Read other articles:

بيوتر جراباركزيك معلومات شخصية الميلاد 31 أكتوبر 1982 (العمر 41 سنة)أولشتين، بولندا الطول 2.00 م (6 قدم 7 بوصة)* مركز اللعب محور [لغات أخرى] الجنسية بولندي الحياة العملية الرقم 2 المهنة لاعب كرة يد الرياضة كرة اليد، وكرة القدم الأندية الاحترافية* الأعوا

Mihrişah SultanSarcofago di Mihrişah SultanValide SultanIn carica7 aprile 1789 –16 ottobre 1805 PredecessoreŞehsuvar Sultan SuccessoreSineperver Sultan Nome completoAgnese (alla nascita) Altri titoliKadın NascitaGeorgia o Genova, 1745 MorteIstanbul, 16 ottobre 1805 SepolturaTürbe Mihrişah Sultan Luogo di sepolturaComplesso Mihrişah Sultan, Eyüp Casa realeCasa di Osman (per matrimonio) Consorte diMustafa III FigliHibetullah SultanSelim IIIFatma Sultan ReligioneIslam sunnit...

العلاقات المغربية البوتسوانية المغرب بوتسوانا المغرب بوتسوانا تعديل مصدري - تعديل العلاقات المغربية البوتسوانية هي العلاقات الثنائية التي تجمع بين المغرب وبوتسوانا.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين: وجه �...

Pemilihan umum Bupati Nganjuk 20182013202327 Juni 2018Kandidat Peta persebaran suara Letak Kabupaten Nganjuk di provinsi Jawa Timur Bupati petahanaAbdul Wachid Badrus (Plt.) Bupati terpilih Novi Rahman Hidhayat PKB, Hanura, PDI-P Sunting kotak info • L • BBantuan penggunaan templat ini Pemilihan umum Bupati Nganjuk 2018 (selanjutnya disebut Pilbup Nganjuk 2018) akan dilaksanakan pada 27 Juni 2018, mengikuti jadwal pilkada serentak gelombang ketiga oleh KPU untuk menentukan Bupat...

Sir Eric Alexander Buchanan, 3rd Baronet (19 August 1848 – 29 July 1928) was the 3rd Baronet Buchanan of Dunburgh.[1] He was born on 19 August 1848. He was the son of Rt. Hon. Sir Andrew Buchanan, 1st Baronet and Frances Katharine Mellish. He was educated at Wellington College, Berkshire. He married Constance Augusta Tennant, daughter of Charles Edmund Tennant RN of Needwood House, Burton-on-Trent, on 27 June 1898. The children from the marriage were: Major Sir Charles James Buchana...

Spreadsheet editor, part of Microsoft 365 Excel redirects here. For other uses, see Excel (disambiguation). Microsoft ExcelA simple bar graph being created in Excel, running on Windows 11Developer(s)MicrosoftInitial releaseNovember 19, 1987; 36 years ago (1987-11-19)Stable release2309 (16827.20130) / September 28, 2023; 2 months ago (2023-09-28)[1] Written inC++ (back-end)[2]Operating systemMicrosoft WindowsTypeSpreadsheetLicenseTrialware ...

Pour les articles homonymes, voir MMX. Martian Moons ExplorationSonde spatiale Vue d'artiste.Données générales Organisation JAXA (principal) CNES et DLR (astromobile) Domaine Étude de Phobos Type de mission Retour d'échantillons Statut En cours de développement Autres noms MMX Lancement Vers 2024 Lanceur H3 Site www.mmx.jaxa.jp Principaux jalons Septembre 2024 Lancement Août 2025 Arrivée dans système martien Août 2028 Départ du système martien Juillet 2029 Retour sur Terre Caract�...

Theory describing the reaction rates of elementary chemical reactions Figure 1: Reaction coordinate diagram for the bimolecular nucleophilic substitution (SN2) reaction between bromomethane and the hydroxide anion In chemistry, transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.[1] TST is used primarily to und...

Jewgeni Lansere (1913): Buchillustration zu Hadschi Murat Hadschi Murat (russisch Хаджи-Мурат, Transkription: Chadschi-Murat, Transliteration: Chadži-Murat) ist eine Erzählung von Lew Tolstoi, die in den Jahren 1896–1906 entstand[1] und 1912 postum erschien. Tolstoi verwendete die Erinnerungen des Unterleutnants und späteren Generals Wladimir Alexejewitsch Poltorazki[2] sowie des Adjutanten und späteren Grafen Michail Loris-Melikow[3]. Dies ist das letz...

Marquesado de Agoncillo Corona marquesalPrimer titular Enrique Frías-Salazar y Torres VildósolaConcesión Alfonso XII de España7 de junio de 1875Actual titular Vacante[editar datos en Wikidata] El marquesado de Agoncillo es un título nobiliario español creado por el rey Alfonso XII el 7 de junio de 1875 en favor de Enrique Frías-Salazar y Torres Vildósola,[1] quien era hijo de Hipólito Frías-Salazar y Sáenz Téllez, y por tanto heredero del Señorío de Agoncillo. S...

Logo dari dua organisasi internasional yang membentuk Komisi Codex AlimentariusOrganisasi Pangan dan Pertanian (FAO)Organisasi Kesehatan Dunia (WHO) Komisi Codex Alimentarius (bahasa Inggris: Codex Alimentarius Commission; disingkat CAC) adalah sebuah organisasi antarpemerintah yang dibentuk bersama oleh Organisasi Pangan dan Pertanian (FAO) dengan Organisasi Kesehatan Dunia (WHO). Komisi ini didirikan pada tahun 1963. Tugas utama dari CAC adalah membuat dan melaksanakan program standar panga...

Historic site in Shanghai, ChinaTomb of LuxunThe tomb of Lu XunLocationLu Xun Park, Shanghai, ChinaCoordinates31°16′27″N 121°28′41″E / 31.27414°N 121.478062°E / 31.27414; 121.478062 (鲁迅墓)Built1956OwnerLu Xun The bronze statue of Lu Xun at his tomb The tomb of Lu Xun is the burial place of the Chinese writer Lu Xun (1881–1936), located in the northwestern corner of the Lu Xun Park in Hongkou District, Shanghai. Covering an area of 1,600 squar...

The Moth Presents: All These Wonders. True Stories About Facing the Unknown First editionEditorCatherine BurnsLanguageEnglishPublisherCrown ArchetypePublication dateMarch 21, 2017Pages336ISBN978-1-101-90440-4WebsiteAll These Wonders The Moth Presents: All These Wonders. True Stories About Facing the Unknown is a 2017 collection of stories from the radio program The Moth, edited by the show's artistic director Catherine Burns on the 20th anniversary of the show's 1997 founding. The 336-page co...

Uniforms of the Bavarian Hartschiere before 1852 Hartschiere[1] (singular form: Hartschier) were predominantly members of the Bavarian residence guards before 1918, a historic military branch of the former Duchy and the later Electorate and at last Kingdom of Bavaria. History According to Meyers Konversations-Lexikon, the Germanized word Hartschier originally derived from the Italian word arciere for archer,[2] but it might also be possible that it has Spanish roots, because t...

You can help expand this article with text translated from the corresponding article in Japanese. (December 2021) Click [show] for important translation instructions. View a machine-translated version of the Japanese article. Machine translation, like DeepL or Google Translate, is a useful starting point for translations, but translators must revise errors as necessary and confirm that the translation is accurate, rather than simply copy-pasting machine-translated text into the English W...

This article may need to be rewritten to comply with Wikipedia's quality standards. You can help. The talk page may contain suggestions. (November 2013) 2011 video gameMonster TaleCover artDeveloper(s)DreamRiftPublisher(s)Majesco EntertainmentDirector(s)Peter OngProgrammer(s)Ryan PijaiArtist(s)Michael VeroniComposer(s)Ian StockerPlatform(s)Nintendo DSReleaseNA: March 22, 2011[1]Genre(s)Action-adventureLife simulation, Metroidvania[2][3]Mode(s)Single-player Monster Tale...

Exhibition pavilion in Vienna, AustriaSecession BuildingSecessionsgebäudeMain facade of the Secession BuildingGeneral informationTypeExhibition pavilionArchitectural styleArt NouveauLocationVienna, AustriaCoordinates48°12′1.86″N 16°21′56.43″E / 48.2005167°N 16.3656750°E / 48.2005167; 16.3656750Construction started1897Completed1898DimensionsDiameter40 m × 30 m (131 ft × 98 ft)Technical detailsFloor area1,000 m2 (11,000&#...

Die mittlere Tiefe dient als Messwert, wenn man z. B. von flachen Gewässern spricht, da sie aussagekräftiger ist als ein einzelner Extrem-Punkt. Die mittlere Tiefe beschreibt die durchschnittliche Distanz zwischen der Wasseroberfläche und dem Grund eines Gewässers. Berechnung Um die mittlere Tiefe von z. B. einem See zu ermitteln, wird das Volumen von ihm durch seine Wasseroberfläche geteilt, sodass der daraus ergebende Quotient einen Durchschnittswert darstellt, der dann als m...
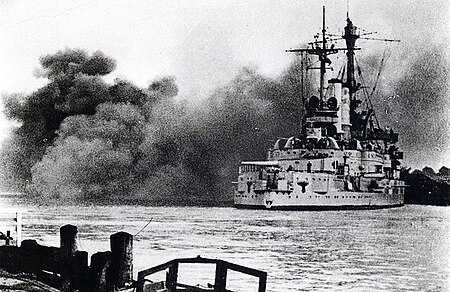
هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (مايو 2018) البارجة الحربية الألمانية شليسفيش هولشتاين تقصف ويستربلات في بولندا في 1 سبتمبر 1939 شهدت الحرب العالميه الثانية نهاية البارجة كقوة مهيمنة في السلاح البح...

لمعانٍ أخرى، طالع النهضة (توضيح). هذه مقالة غير مراجعة. ينبغي أن يزال هذا القالب بعد أن يراجعها محرر؛ إذا لزم الأمر فيجب أن توسم المقالة بقوالب الصيانة المناسبة. يمكن أيضاً تقديم طلب لمراجعة المقالة في الصفحة المخصصة لذلك. (يوليو 2021) هذه المقالة يتيمة إذ تصل إليها مقال�...







