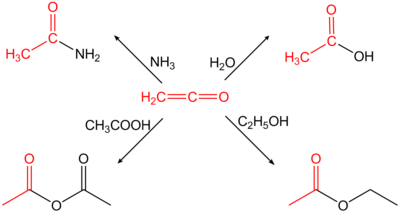
Ethenone
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Cinema of Pakistan List of Pakistani films Pakistani Animation Highest Grossing Pre 1950 1950s 1950 1951 1952 1953 19541955 1956 1957 1958 1959 1960s 1960 1961 1962 1963 19641965 1966 1967 1968 1969 1970s 1970 1971 1972 1973 19741975 1976 1977 1978 1979 1980s 1980 1981 1982 1983 19841985 1986 1987 1988 1989 1990s 1990 1991 1992 1993 19941995 1996 1997 1998 1999 2000s 2000 2001 2002 2003 20042005 2006 2007 2008 2009 2010s 2010 2011 2012 2013 20142015 2016 2017 2018 2019 2020s 2020 2021 2022 20...

English aristocrat Elizabeth StaffordDuchess of NorfolkBornc. 1497Died30 November 1558BuriedChurch of St Mary-at-Lambeth, SurreyNoble familyStafford (by birth)Howard (by marriage)Spouse(s)Thomas Howard, 3rd Duke of NorfolkIssueHenry Howard, Earl of SurreyLady Mary Howard, Duchess of Richmond and SomersetThomas Howard, 1st Viscount Howard of BindonLady Muriel HowardLady Katherine Howard, Countess of DerbyFatherEdward Stafford, 3rd Duke of BuckinghamMotherLady Eleanor Percy Lady Elizabeth...

Pour les articles homonymes, voir Attias. Richard AttiasRichard Attias en 2011.BiographieNaissance 19 novembre 1959 (64 ans)FèsNationalité marocaineFormation Institut national des sciences appliquées de ToulouseActivités Homme d'affaires, publicitaire, modérateur de débatConjoint Cécilia Attias (depuis 2008)modifier - modifier le code - modifier Wikidata Richard Attias est un homme d'affaires marocain[1] né à Fès[2] le 19 novembre 1959. Publicitaire, Richard Attias est connu po...

XQD Тип носія Карта пам'ятіЄмність До 240 ГБРозроблено CompactFlash AssociationРозміри 38.5 mm × 29.8 mm × 3.8 mmЗастосування Цифрові камери XQD-картрідер Sony XQD — формат карт пам'яті, призначений для особливо вимогливих до швидкості читання і запису пристроїв обробки цифрових даних.

The King Uzziah Stricken with Leprosy, by Rembrandt, 1635. Uzia (Ibrani: עֻזִּיָּהוּ, YHWH adalah kekuatanku; bahasa Yunani: Οζίας; bahasa Latin: Ozias); bahasa Inggris: Uzziah), atau yang dikenal juga dengan nama Azarya (Ibrani: עֲזַרְיָה, YHWH telah menolong; bahasa Yunani: Αζαρις; bahasa Inggris: Azariah) adalah raja ke-10 kerajaan Yehuda (791 SM - 739 SM). Ayahnya adalah raja Amazia[1][2] dan ibunya bernama Yekholy...

I Putu Sukreta SurantaAnggota Dewan Pertimbangan AgungMasa jabatan13 Juni 1998 – 31 Juli 2003PresidenB.J. HabibieAbdurrahman WahidMegawati SukarnoputriInspektur Jenderal Departemen Pertahanan dan KeamananMasa jabatan24 April 1993 – 1998Pendahulutidak diketahuiPenggantiFarid ZainuddinAnggota Majelis Permusyawaratan RakyatMasa jabatan1 Oktober 1992 – 1 Juli 1998PenggantiDavid Napitupulu Informasi pribadiLahir(1938-04-11)11 April 1938Klungkung, Bali, Hindia Belan...

El próximo Rembrandt (en inglés: The Next Rembrandt) es una pintura elaborada gracias a la tecnología 3D e información de la técnica y forma de trabajo del pintor barroco neerlandés Rembrandt Harmenszoon van Rijn. La elaboración del proyecto corrió a cargo de la agencia holandesa J. Walter Thompson Amsterdam que logró el objetivo por medio de algoritmos profundos de aprendizaje y técnicas de reconocimiento facial[1][2]. Proceso de elaboración Para hacer posible el pro...

K computer K computer — японський суперкомп'ютер, створений компанією «Fujitsu» для одного з інститутів Riken[en] у Кобе, префектура Хьоґо. Уперше запущений у 2011 році.[1][2][3] Названий японський словом «kei» (яп. 京), що означає 10 квадрильйонів (1016).[1] 20 липня 2011 року організа

Hans KammlerFoto ID NSDAP, 1932Lahir(1901-08-26)26 Agustus 1901Stettin, Kekaisaran JermanMeninggal9 Mei 1945(1945-05-09) (umur 43)[a]dekat PraguePengabdian Nazi GermanyDinas/cabangSSPangkatSS-Obergruppenführer und General der Waffen-SSKomandanKantor D di dalam Kantor Ekonomi dan Administrasi Utama SS Hans Kammler (26 Agustus 1901 – 9 Mei 1945) adalah seorang perwira SS-Obergruppenführer yang bertanggung jawab atas proyek teknik sipil dan program senjata rah...

1937 Romanian general election ← 1933 20 December 1937 (1937-12-20) 1939 → All 387 seats in the Chamber of DeputiesAll 113 seats in the SenateTurnout66.07% First party Second party Leader Dinu Brătianu Iuliu Maniu Party PNL PNȚ Leader since 1934 1937 Last election 105 S / 300 D 0 S / 29 D Seats won 97 S / 152 D 10 S / 86 D Seat change 8 S / 148 D 10 S / 57 D Popular vote 1,103,353 D 626,612 D Percentage 36.46% D 20....

Douze variations en do majeur pour piano sur « Ah ! vous dirai-je, maman » KV 265/300e Début de la partition. Genre Variations pour piano Nb. de mouvements thème et 12 variations Musique Wolfgang Amadeus Mozart Durée approximative Env. 12 min Dates de composition 1781 ou 1782 Partition autographe Autographe fractionné entre plusieurs bibliothèques Fichier audio Ah ! vous dirai-je, maman noicon265/300e par Stefano Ligoratti modifier Les douze variations en...

US anti-drug campaign This article is about the campaign. For the episode of The Riches, see This Is Your Brain On Drugs (The Riches). The Partnership used a simple advertisement showing an egg in a frying pan, similar to this photo, suggesting that the effect of drugs on a brain was like an egg on a hot pan. This Is Your Brain on Drugs was a large-scale US anti-narcotics campaign by Partnership for a Drug-Free America (PDFA) launched in 1987, that used three televised public service announce...

Telephone numbers in SwedenLocation of Sweden in dark greenLocationCountrySwedenContinentEuropeRegulatorSwedish Post and Telecom AuthorityTypeOpenNSN length7 to 13 digitsNumbering planSweden National Numbering PlanLast updated22 May 2018Access codesCountry code+46International access00Long-distance0 In Sweden, the area codes are, including the leading 0, two, three or four digits long, with larger towns and cities having shorter area codes permitting a larger number of telephone numbers in th...

Pour les articles homonymes, voir Statue de Jeanne d'Arc. Statue de Jeanne d'ArcLa statue devant l'hôtel de ville de Vaucouleurs.PrésentationType StatueFondation 1946Créateur Georges Halbout du TanneyMatériau bronzeLocalisationLocalisation Vaucouleurs, Meuse FranceCoordonnées 48° 36′ 06″ N, 5° 39′ 57″ Emodifier - modifier le code - modifier Wikidata Le monument à Jeanne d'Arc est une statue équestre en bronze représentant Jeanne d'Arc en ar...

Chinese TV series or program Love Yunge from the DesertGenreHistorical fictionRomanceBased onSong in the Clouds by Tong HuaWritten byShen ZhiningDirected byHu YijuanCai ChangshengHu MingkaiStarringAngelababyDu ChunLu YiChen XiaoYang RongOpening themeSilk by Li YuchunEnding themeGreen Skirt by AngelababyCountry of originChinaOriginal languageMandarinNo. of episodes45ProductionExecutive producerYu ZhengProduction locationHengdian World StudiosProduction companiesDongyang XingruiDongyang Hu...
Опис файлу Обґрунтування добропорядного використання для статті «Красуня на всю голову» [?] Опис Постер до фільму «Красуня на всю голову», 2018 Джерело imdb.com Час створення невідомо Мета використання Ілюстрація предмета статті Замінність Заміна вільним файлом немож...

Основные статьи: Культура СССР и Научная фантастика § НФ в России и СССР Космическая фантастика. Спутник внеземной цивилизации, художник Андрей Соколов, почтовая марка СССР, 1967 Советская фантастика — произведения писателей в фантастическом жанре, творчество котор...

Населення Косова Ріст чисельності населення країниЧисельність 1,87 млн осіб осібКоефіцієнт міграції -3,7 ‰Природний рухПриродний приріст 6,4 %Народжуваність 17,1 ‰Фертильність 2,09 дітей на 1 жінкуСмертність 7,0 ‰Смертність немовлят 36,4 ‰Вікова структура ...

Tax-free zone in Dubai, UAE This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (January 2016) (Learn how and when to remove this template message) Dubai International Media CityView of the skyscrapers in the Dubai Media City areaTypeFree Economic ZoneFounded2000; 23 years ago (2000)HeadquartersDubai, United Arab EmiratesKey peopleMajed Al Suwaid...

Beker van België 2016-17 kan verwijzen naar: Beker van België 2016-17 (mannenvoetbal) Beker van België 2016-17 (vrouwenvoetbal) Bekijk alle artikelen waarvan de titel begint met Beker van België 2016-17 of met Beker van België 2016-17 in de titel. Dit is een doorverwijspagina, bedoeld om de verschillen in betekenis of gebruik van Beker van België 2016-17 inzichtelijk te maken. Op deze pagina staat een uitleg van de verschillende betekenissen van Beker van Belgi�...