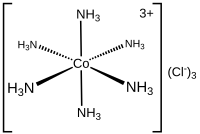
Coordination number
|
Read other articles:

Hengchun 恆春Kota kecil urbanKota kecil Hengchun· 恆春鎮Jalan Utama Kenting (Pasar Malam Kenting), HengchunKota kecil Hengchun dalam Kabupaten PingtungNegaraTaiwanRegionTaiwan SelatanLuas • Total136,7630 km2 (528,040 sq mi)Populasi (Desember 2014) • Total30.859 • Kepadatan0,023/km2 (0,058/sq mi)Rumah tangga10568Divisi17 desa, 272 lingkunganKode pos946 Nama TionghoaHanzi Tradisional 恆春鎮 Arti harfiahKota Musim Semi Ab...

Humboldt County County in de Verenigde Staten Situering Staat Iowa Coördinaten 42°46'38NB, 94°12'15WL Algemeen Oppervlakte 1.128 km² - land 1.125 km² - water 3 km² Inwoners (2000) 10.381 (9 inw./km²) Overig Zetel Dakota City FIPS-code 19091 Opgericht 1851 Detailkaart Overzichtskaart van Homboldt County Foto's Bevolkingspiramide Humboldt County Statistieken volkstelling Humboldt County Portaal Verenigde Staten Humboldt County is een county in de Amerikaanse staat Iowa. De c...

Mendelsohn kan verwijzen naar: Carol Mendelsohn, een Canadese tv-schrijfster Erich Mendelsohn, een Duits architect Zie ook Mendelssohn Bekijk alle artikelen waarvan de titel begint met Mendelsohn of met Mendelsohn in de titel. Dit is een doorverwijspagina, bedoeld om de verschillen in betekenis of gebruik van Mendelsohn inzichtelijk te maken. Op deze pagina staat een uitleg van de verschillende betekenissen van Mendelsohn en verwijzingen daarnaartoe. Bent u hier via e...

Minskin Asal Amerika Serikat Standar ras TICA standar Kucing domestik (Felis catus) Minskin adalah salah satu ras kucing baru dan sangat langka yang merupakan persilangan dari ras munchkin dengan sfinks. Ciri khasnya adalah berkaki pendek dan tidak memiliki bulu.[1] Sejarah Pada tahun 1998, seorang peternak kucing bernama Paul McSorley menyilangkan ras munchkin dengan ras sphynx. Setelah penyilangan, lahir ras kucing baru yang mirip dengan ras sfinks, tetapi berkaki pendek seper...

Agrigulture policy proposal by Huey Long A South Carolina cotton field in 1932 This article is part of a series aboutHuey Long Political views Early life Early career In culture Governor of Louisiana 1924 campaign 1928 election State Capitol Cotton-Holiday U.S. Senator from Louisiana Tenure Chaco War Share Our Wealth Assassination American Progress Every Man a King (book) Every Man a King (song) My First Days in the White House vte The Cotton-Holiday was a 1931 proposal by Louisiana Governor ...

English actress (1878–1975) Ethel GriffiesGriffies in Jane Eyre (1943)BornEthel Woods(1878-04-26)26 April 1878Sheffield, West Riding of Yorkshire, EnglandDied9 September 1975(1975-09-09) (aged 97)London, EnglandOccupationActressYears active1881–1967Spouses Walter Beaumont (m. 1900; died 1910) Edward Cooper (m. 1917; died 1956) Ethel Griffies (born Ethel Woods; 26 April 1878 �...

ДеревняСанницы 55°59′30″ с. ш. 43°03′58″ в. д.HGЯO Страна Россия Субъект Федерации Нижегородская область Муниципальный район Павловский Городское поселение Рабочий посёлок Тумботино История и география Часовой пояс UTC+3:00 Население Население ↗208[1] челове...

Pakistani politician This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Muhammad Dilawar Khanji – news · newspapers · books · scholar · JSTOR (September 2015) (Learn how and when to remove this template message) Muhammad Dilawar Khanjiમુહમ્મદ દિલાવર ખાનمحمد دلاور خان ...

東京都精神医学総合研究所 東京都精神医学総合研究所正式名称 東京都精神医学総合研究所英語名称 Tokyo Institute of Psychiatry略称 精神研組織形態 財団法人所在地 日本〒156-8506東京都世田谷区上北沢 2-1-6設立年月日 1973年7月プロジェクト 精神疾患研究分野社会精神医学研究分野精神生物学研究分野基盤技術部門ウェブサイト http://prit.igakuken.or.jp/index.htmlテンプレートを表示 ...

Syrian political family Al-Assad redirects here. For other uses, see al-Assad (disambiguation). Al-Assad Familyعَائِلَة الْأَسَدʿāʾilat al-ʾAsadThe Assad family, c. 1993. Front: Hafiz al-Assad and his wife, Anisa Makhlouf. Rear, left to right: Maher, Bashar, Bassel, Majid, and Bushra al-AssadCurrent regionLatakiaPlace of origin SyriaMembersHafez al-Assad Bashar al-Assad Maher al-Assad Rifaat al-AssadConnected familiesMakhlouf, Shalish The al-Assad family,[a&...

German television host, producer and actor Joko WinterscheidtWinterscheidt at the Grimme Awards 2018BornJoachim Winterscheidt (1979-01-13) 13 January 1979 (age 44)Mönchengladbach, West Germany[1]Occupations Television host producer Years active2005–presentChildren1AwardsSee Awards Joachim Joko Winterscheidt (born 13 January 1979) is a German television host, producer and actor. He became known as part of the duo Joko & Klaas alongside Klaas Heufer-Umlauf in TV program...

Samanar MalaiSamanar HillsReligionAffiliationJainism, HinduismLocationLocationKeelakuyilkudi village, Tamil NaduArchitectureDate established2000 years oldCompleted9th century Part of a series onJainism Jains History Timeline Index Philosophy Anekantavada Cosmology Ahimsa Karma Dharma Mokṣa Kevala Jnana Dravya Tattva Brahmacarya Aparigraha Gunasthana Saṃsāra EthicsEthics of Jainism Mahavratas (major vows) Ahiṃsā (non-violence) Satya (truth) Asteya (non-stealing) Brahmacarya (chastity) ...

Artikel ini sudah memiliki daftar referensi, bacaan terkait, atau pranala luar, tetapi sumbernya belum jelas karena belum menyertakan kutipan pada kalimat. Mohon tingkatkan kualitas artikel ini dengan memasukkan rujukan yang lebih mendetail bila perlu. (Pelajari cara dan kapan saatnya untuk menghapus pesan templat ini) Artikel ini membutuhkan rujukan tambahan agar kualitasnya dapat dipastikan. Mohon bantu kami mengembangkan artikel ini dengan cara menambahkan rujukan ke sumber tepercaya. Pern...

Il centro di San Giovanni Valdarno La storia dell'urbanistica è la disciplina che studia l'evoluzione degli insediamenti umani, a partire dall'antichità fino agli sviluppi contemporanei e contemporaneamente l'evoluzione dell'urbanistica come attività e disciplina che ha come fine la pianificazione, il riordinamento, il risanamento, l'adattamento funzionale di aggregati urbani già esistenti e la progettazione di nuovi aggregati.[1] Indice 1 Definizione 2 Civiltà antiche 3 Urbanist...

Cuban historian (1942–2020) Eusebio LealBornEusebio Leal Spengler(1942-09-11)11 September 1942Havana, CubaDied31 July 2020(2020-07-31) (aged 77)Havana, CubaCitizenshipCubanAlma materUniversity of HavanaOccupationHistorianKnown forRestoration of Old Havana Eusebio Leal Spengler (11 September 1942 – 31 July 2020) was a Cuban historian. He served as the municipal historian of Havana, as well as the director of the restoration project of Old Havana. Under his overs...

Respect and appreciation of Jewish people You can help expand this article with text translated from the corresponding article in German. (November 2021) Click [show] for important translation instructions. Machine translation, like DeepL or Google Translate, is a useful starting point for translations, but translators must revise errors as necessary and confirm that the translation is accurate, rather than simply copy-pasting machine-translated text into the English Wikipedia. Consider ...

American computer programmer Brian Jhan FoxFox 2008Born (1959-12-11) December 11, 1959 (age 63)Boston, Massachusetts, U.S.OccupationsComputer programmertechnologistauthorEmployer(s)Opus Logica, Inc.Known forGNU BashRelativesDonal Fox (brother) Brian Jhan Fox (born 1959) is an American computer programmer and free software advocate. He is the original author of the GNU Bash shell, which he announced as a beta in June 1989.[1] He continued as the primary maintainer of bash unt...

P. Chester Beatty I, ( p {\displaystyle {\mathfrak {p}}} 45) folii 13-14, contenenti una porzione del Vangelo secondo Luca I Papiri Chester Beatty sono una collezione di 11 antichi manoscritti biblici su papiro, composti in lingua greca e di origine cristiana. Sette manoscritti contengono porzioni dell'Antico Testamento, tre del Nuovo Testamento e uno contiene il Libro di Enoch e un'omelia cristiana non identificata. Sono per lo più datati al III secolo e conservati in parte alla Chester Bea...

Ini adalah nama Tionghoa; marganya adalah Soo. Carmen SooAktor Melayu Carmen Soo dari Oh My English! diwawancarai di MeleTOP, 2015LahirSoo Wai Ming14 Oktober 1977 (umur 46)Kuala Lumpur, MalaysiaPekerjaanModelaktrisPemandu acara TVTahun aktif1997–sekarangInformasi modelingTinggi156 cm (5 ft 1 in) Situs webcarmensoo.com Soo Wai Ming (Hanzi sederhana: 苏慧敏; Hanzi tradisional: 蘇慧敏; Pinyin: Su Huimin; (lahir 14 Oktober 1977) adalah seorang model da...

K-Beauty es un término general para los productos para el cuidado de la piel que se derivan de Corea del Sur. La moda ganó popularidad en todo el mundo, especialmente en el este de Asia, el sudeste de Asia, el sur de Asia y el mundo occidental, y se centra en la salud, la hidratación y el énfasis en los efectos iluminadores.[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16]...